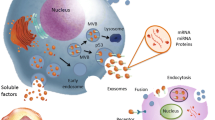
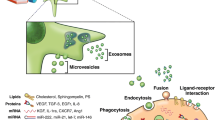
Abstract
Mesenchymal stromal cells (MSCs) as a kind of pluripotent adult stem cell have shown great therapeutic potential in relation to many diseases in anti-inflammation and regeneration. The results of preclinical experiments and clinical trials have demonstrated that MSC-derived secretome possesses immunoregulatory and reparative abilities and that this secretome is capable of modulating innate and adaptive immunity and reprograming the metabolism of recipient cells via paracrine mechanisms. It has been recognized that MSC-derived secretome, including soluble proteins (cytokines, chemokines, growth factors, proteases), extracellular vesicles (EVs) and organelles, plays a key role in tissue repair and regeneration in bronchopulmonary dysplasia, acute respiratory distress syndrome (ARDS), bronchial asthma, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), pulmonary arterial hypertension, and silicosis. This review summarizes the known functions of MSC-EV modulation in lung diseases, coupled with the future challenges of MSC-EVs as a new pharmaceutical agent. The identification of underlying mechanisms for MSC-EV might provide a new direction for MSC-centered treatment in lung diseases.
Graphical abstract

Similar content being viewed by others
Introduction
Respiratory diseases are leading causes of morbidity and mortality worldwide as the lung is a vital and vulnerable organ that is exposed to the ubiquity of pollutional environmental, occupational, and behavioural inhalational exposures [1, 2]. According to an analysis for the global burden of disease study (GBD) 2017, more than 500 million people in the world had a chronic respiratory disease and these diseases accounted for approximately 4 million deaths in 2017 [3]. Regarding acute lung injury (ALI), patients with ARDS occupy 10% of all beds in intensive care units (ICU) and the mortality rate for ARDS remains between 30% to 40% in most clinical research [4]. Whatever the pathophysiology of acute lung injury or chronic respiratory diseases, the overwhelming immune responses, and inappropriate reparative processes usually result in an imbalance of pro-inflammatory and anti-inflammatory cytokines, and profibrotic and anti-fibrotic factors, which give rise to irreparable damage and exert a negative impact on the quality of life [5]. The current therapies for acute lung injury and most chronic lung diseases remain in the areas of anti-inflammation and corticosteroid treatment, which have potential side effects and uncertain outcomes [6] such as increased risk of pneumonia, oral candidiasis, tuberculosis, etc. Over the past decades it has become clear that MSCs, regarded as “the Next Pillar of Medicine”, are able to restore the balance of the immune response in the process of pulmonary inflammation by modulating the cytokine network and other humoral and cellular effectors. They have been identified as having not only profound immunosuppressive effects but have also demonstrated an ability to facilitate wound healing in acute or chronic lung injury. However, mounting evidence demonstrates that only a small number of MSCs are capable of preferentially homing to damaged places and surviving for over 24 h through systematic administration [7]. Moreover, the ambiguous impacts of MSC administration, such as emboli formation and tumorigenic transformation, genetic instability, and the lack of standardized and optimized criteria contribute to the investigation of MSC-EVs as an alternative agent. Most importantly, in some experimental lung disease models, MSC-EVs obtained more effective outcomes in terms of lung vascularization and alveolarization compared to MSCs [8,9,10].
In this review, we pay attention to recent insights from preclinical experiments and clinical trials which have contributed to dissect the molecular mechanisms of MSC-EV effects, and highlight the existing barrier of MSC-EV application as an off-shelf agent from bench to bedside for lung diseases (Table 1).
MSC-EVs: Definition, Characteristics and Potential Effects in Lung Diseases
MSC-EVs are round signal molecules delimited by a lipid bilayer membrane, which play a prominent role in extracellular communications through delivering parental cell-derived active cargos such as bioactive proteins, mRNAs, noncoding RNAs and organelles to recipient cells [11]. Based on the Minimal information for studies of Extracellular Vesicles 2018 (MISEV2018), the International Society for Extracellular Vesicles (ISEV) suggests a new nomenclature of MSC-EVs subtypes, which is based on: 1) physical characteristics of EVs; 2) biochemical composition; or 3) descriptions of conditions or cell of origin [12]. However, most studies currently still use the general classification for MSC-EV subtypes: exosomes (30-120 nm), microvesicles (100-1000 nm), and apoptotic bodies (800-5000 nm) [13]. Recently, research on EV biogenesis and shedding has indicated that exosomes and microvesicles have two different secretory mechanisms, of which exosomes are derived and generated through endocytic pathway, and subsequently fuse either with lysosomes or with plasma membrane [14, 15]. By contrast, microvesicles are originated by plasma membrane budding and release directly from the cell surface [16]. Furthermore, the contents of the EV cargo are dependent on the type of their parental cells and the microenvironment of the releasing cells [17, 18].
The lungs are the primary organ of the respiratory system that are in contact with the external environment containing pathogens and microbes. Pulmonary homeostasis is maintained by the communication between local stromal cells and resident immune cells that sense the dynamic microenvironment. Upon the disruption of homeostasis by risk pathogens, resident macrophages as the first line of defense against various pathogenic microorganisms and the primary source for the release of proinflammatory cytokines and chemokines, such as TNF-α, IL-1β, and macrophage inflammatory protein-2(MIP-2), recruit neutrophils and monocytes respectively to propagate the immune responses [19]. In an extremely uncontrolled microenvironment, excessive inflammation, aberrated immunomodulation or unknown etiologies call for clinical intervention. To date, antibiotics, corticosteroid, and invasive ventilation are primary choice for treating respiratory disease, but multi-drug resistance, opportunistic infection, and unrecoverable injury are main side effects to patients. In recent years, compared to the pharmacological treatments, MSC-EVs have exhibited immunosuppressive and reparative properties in the way of low immunogenicity, long half-life, in vivo stability, and high delivery efficiency, which also contribute to attenuate lung injury and facilitate wound closure. Accordingly, employing MSC-EVs is likely to be a promising approach due to their ability to reduce lymphocyte infiltration and pro-inflammatory cytokine secretion, inhibit bacteria or virus replication, regulate endothelial and epithelial permeability, and promote tissue repair [20,21,22]. Moreover, accumulating data have shown MSC-EVs are capable of modulating proliferation, maturation, polarization, and migration of different immune effector cells depending on the context of delivering various cytokines, transcription mediators, and organelles, which contribute to the preferential characteristics of MSC-EVs in their immunomodulatory effects [23, 24].
Molecular Mechanisms of MSC-EVs in Lung Diseases
Bronchopulmonary Dysplasia
Bronchopulmonary dysplasia (BPD) is a chronic respiratory disease most commonly seen in preterm infants and neonates who require mechanical ventilation and oxygen therapy for acute respiratory distress [25], characterized by a dysregulated immune response, decreased numbers of alveoli and blood vessels, and dysfunction of the aveolar-capillary membrane [26]. In preclinical studies, newborn mice or rats exposed to a hyperoxia (75%) microenvironment are widely used to mimic the pathogensis of human BPD [27]. Over the years, systematic administration, or local injection (intranasal [28] or intratracheal [29]) of MSCs have defined the beneficial impact on attenuating experimental BPD through inhibition of N-methyl-D-aspartic acid (NMDA) receptors [30], renin-angiotensin system (RAS) [31], TLR4 expression [32], decorin [29] and CTGF secretion [33], accompanied by upregulating the production of aminoacyl-peptide hydrolase [34], PTX3 [35], VEGF [33], stromal cell-derived factor 1 [36], macrophage stimulating factor 1 [37], and osteopontin [37], leading to increased survival rate, downregulated inflammation- and hyperoxia-induced defective alveolarization, and reduced lung fibrosis in experimental BPD mice. Moreover, MSC stably transfected with a truncated version of CC chemokine ligand 2 (CCL2) promotes macrophage activation, and is seen to be more effective than MSCs alone [38]. These promising preclinical data have contributed to the application of MSCs in clinical trials (Table 2). For example, Chang et al launched a phase I dose-escalation trial (NCT01297205) of hUCB-derived MSC transplantation in BPD, recruiting 9 preterm infants of which three were given a low dose (1*107 cells/Kg) and the other six were administrated a high dose (2*107 cells/Kg). Both groups showed that MSC administration in treating BPD in preterm infants is safe and feasible [39].
Nevertheless, safety concerns regarding the transplantation of MSC in newborns have facilitated the investigation of MSC-EV effects in BPD. Willis et al conducted pioneering research to assess the efficacy of MSC-exo treatment in an experimental hyperoxia-induced BPD model and to investigate mechanisms underlying the therapeutic effect [40]. They have demonstrated that MSC-exos administrated intravenously at the concerntration of 8.5*108 particles/50ul improve pulmonary development, ameliorate septal fibrosis, restore lung architecture, and enhance peripheral pulmonary arterial remodeling through macrophage phenotype modulation [40]. Noteworthily, the other studies done by Braun and Porzionato et al have also shown that MSC-EV injection intraperitoneally(3.4*109/50ul) or intratracheally(6.4*109 EVs/50ul) increases blood vessel number and lung size, prevents right heart hypertrophy, and inhibits alveolar growth disruption via anti-inflammatory and pro-angiogenic mechanisms [8, 41]. Moreover, MSCs transferred exosomal factor-TSG-6 partially restores the alveolar-capillary leakage, increasing chord length and alveolar simplification in hypoxia-induced neonatal BPD mouse models [42].There is an ongoing clinical trial (NCT03857841) aiming to investigate the intravenous infusion of BM-MSC-drived EV (UNEX-42) on preterm neonates at high risk for BPD. This interventional, randomized, and placebo-controlled phase I clinical trial will recruit 18 infants and has three dose arms: 20/40/60 pmol phospholid/Kg body weight. Collectively, MSC-EV administration holds great therapeutic potential for BPD by facilitating macrophage polarization, improving alveolarization and angiogenesis, and reducing collagen density in the experimental studies of BPD. (Fig. 1).
Therapeutic effects of MSC-EVs in BPD. MSC-exosomes deliver biological molecules to modulate macrophage polarization, shifting into an anti-inflammatory (M2) phenotype. TSG-6 packaged in MSC-EVs downregulates neutrophil infiltrates, alveolar-capillary leak, septal thickness, and PH-induced RVH. Arg-1 mRNAs wrapped in MSC-EVs promote total lung capacity and alveolar simplification and decrease the production of pro-inflammation cytokines (CCL2, CCL7, IL-6, TNF-α). Meanwhile, MSC-EVs deliver VEGF to damaged tissue to regulate alveolar growth and lung blood vessel density.
Acute Respiratory Distress Syndrome and Severe Pneumonia
Acute Respiratory Distress Syndrome (ARDS) is a form of severe hypoxemic respiratory failure caused by several risk factors, such as pneumonia, sepsis, and trauma, which is characterized by diffuse alveolar damage (DAD) with apoptosis of alveolar type I and II cells, accumulation of proteinaceous oedema, and hyaline membrane formation in the alveolar space [43]. Since the definition of ARDS was established 50 years ago, there has been remains no specific pharmacological treatment for ARDS. Data from preclinical experiments have shown that MSCs prevent the development of ARDS in vitro and in vivo in the experimental acute lung injury (ALI)/ARDS mice models which are instilled with lipopolysaccharide (LPS) or bacteria. MSCs have been reported to secrete various kinds of paracrine factors to restore epithelial and endothelial cell permeability (Ang1, IL-1ra, PGE2, HGF) [44,45,46], facilitating macrophage phagocytosis (IL-6, PGE2) [47], downregulating acute inflammation (IL-1ra, TSG-6, IGF-1, Lipoxin A4) [48], and improving alveolar fluid clearance (KGF7) [49, 50]. Importantly, early clinical trials (phase I and phase II a/b) suggest that it is safe to give MSCs to patients with ARDS [51], and the MUST-ARDS study conducted by Athersys Inc. with a patented bone marrow-derived adult multipotent progenitor cell product (MultiStem) reported a significant reduction in 28-day mortality accompanied by an increase in both ventilator and ICU free days in patients who had received cell therapy [52]. More recently, systematic MSC administration has shown its outstanding properties in improving clinical symptoms and modulating immune responses in critically ill COVID19-ARDS patients [53, 54].
Similarly, MSC-EVs have been shown to be beneficial to experimental ARDS in viral- (H5N1, H1N1), bacterial-(Escherichia coli.), and LPS-induced acute lung injury (ALI). In an influenza A (H5N1)–induced ALI, umbilical cord derived MSC-exosomes at the concentration of 1*1010 particles/90ul have been shown to be more effective in improving alveolar fluid clearance and attenuating protein permeability of alveolar epithelial cells than UC-MSCs due to their greater production of Ang1 and HGF. Moreover, PKH-26-labeled MSC-EVs are able to merge with epithelial cells to suppress virus replication, virus shedding, virus-induced apoptosis, and hemagglutination activity in the other influenza-induced ALI porcine model [55]. Additionally, in an E.coli pneumonia-mediated ALI murine model, Monsel and colleagues have elucidated that human MSC-derived microvesicles (MVs) administration (97 ± 90 ng protein/90ul) decreases the influx of inflammatory cells, and the level of cytokines, protein, and bacteria, coupled with the increased intracellular ATP production in damaged alveolar epithelial type 2 cells, partially through KGF secretion [56]. Most of the studies used an endotoxin (LPS)-induced animal model to mimic the human ARDS/pneumonia microenvironment and to evaluate the MSC-EV effects. Noteworthily, miRNAs packaged in MSC-EVs have been found to play a key role in attenuating ARDS lung injury. It has been reported that MSC-EVs delivered miR21-5p [57]/miR30b-3p [58]/ miR100 [59]/ miR145a [60] /miR146a [61] to attenuate the inflammatory responses. Moreover, MSC-EVs also transferred miR27a-3p / miR146a to modulate macrophage polarization [59, 62]. Besides miRNAs, Zhu et al and Tang et al have demonstrated that MSC-EVs (30.9 ± 17.0μg protein/30ul or isolated from 3*106 MSCs/30ul) are capable of reducing inflammatory cell influx, protein permeability, MIP2 production, extravascular lung water, and increasing IL-10 secretion in the LPS-induced mouse models [63, 64]. More importantly, in the ex vivo perfused human lung models, Gennai et al and Park et al have shown that MSC-microvesicles (16.5μg/200ul or 9.4 ± 0.2*107 particles/200ul) are able to decrease pulmonary artery pressure and resistance, lactate elevation, bacterial CFU, lung protein permeability and lung weight, and increase alveolar fluid clearance (AFC) rate, lung compliance and antimicrobial effect [65, 66]. MSCs also donate functional mitochondria to macrophages to modulate macrophage polarization through enhancement in oxidative phosphorylation and to improve mitochondria function of epithelial cells, resulting in wound closure in a clinically relevant model of ARDS [67, 68].
A pilot clinical trial (NCT04276987) regarding aerosol inhalation of allogenic adipose MSC-exosomes for treating COVID in critically ill patients has been completed by Rujin Hospital, Shanghai. This forerunning phase I trial recruited 24 participants who received conventional treatment and five times aerosol inhalation of MSC-exosomes (2*108 nano vesicles at day1,2,3,4,5), but no results have been posted to date.
Bronchial Asthma
Bronchial asthma is one of the most common and chronic lung diseases in children and adults, whose pathophysiology is underpinned by a chronic inflammation of the airway walls accompanied by mucus hypersecretion, epithelial shedding, metaplasia and hyperplasia of goblet cells, increased collagen deposition, and hypertorophy and hyperplasia of airway smooth-muscle [69, 70]. The current treatments for asthmatic patients are largely symptomatic and ineffective, and diverse side effects of these therapies has led researchers and clinicians to seek safe and effective candidates for this chronic disease. Studies into the effect of MSC on bronchial asthma have shown great potential in attenuating the major pathologic characteristics of asthma including airway immune responses, hyperresponisveness, and remodelling.
Boldrini-Leite et al have found that in the ovalbumin (OVA)-induced asthma BALB/c mice model, the MSC-treated group is able to reduce the amount of eosinophil, lymphocyte, total protein, H2O2, IL-5, IL-13 and IL-17a in the BALF [71]. Similarly, Abreu et al and Song et al have demonstrated that MSCs are capable of reducing lung inflammation and tissue remodeling through promoting the production of anti-inflammatory cytokines and angiogenic factors (IL-4, IL-13, TGF-β, VEGF, VCAM-1, ICAM-1) [72, 73], activating TGF-β signaling to induce M2-like macrophage polarization [74], decreasing oxidative stress, the thickness of basement membrane, epithelium, subepithelial and smooth muscle layer as well as the number of mast cells and goblet cells [75,76,77]. Moreover, in house dust mite-induced allergic asthma, MSCs attenuate the secretion of epithelial cell-derived alarmins IL-ra, pro-Th2 cytokine IL-25, and the number of activated and antigen-acquiring CD11c + CD11b + dendritic cells [78, 79]. Additionally, MSC-conditional medium has also shown beneficial effects in decreasing pathologic scores of the OVA-injuried lung by elevating the mRNA expression of T-bet and IFN-γ, while decreasing the GTAT3 mRNA expression [80]. Unfortunately, although the preclinical evidence has shown the beneficial effects in asthema and several clinical trials in asthmatic patients are ongoing, there remains no data of clinical trials published to date [81].
Compared to MSCs, MSC-EVs (50ul) isolated from 1*105 AD-MSCs show a similar capacity to reduce lung inflammation and a reversal of injured tissue remodelling in the OVA-induced (20μg) asthmatic model by reducing the amounts of eosinophils, collagen fiber in airways, TGF-β production in lung tissue, and CD3 + CD4+ T cells counts in the thymus [82], and also promote the proliferation and immunosuppressive ability of Treg cells [83]. Fang and colleagues have demonstrated that MSC-sEVs (2 × 1010 sEVs/100μg protein) exhibit a significant impact in inhibiting inflammatory cell infiltration, airway hyper-responsiveness, mucus secretion, and downregulating T help 2 cytokines and the function of group 2 innate lymphoid cells (ILC2s), More specifically, miR146a-5p packaged in the sEVs has been revealed to mediate the above effects to rejuvenate ILC2s-dominant allergic airway injury [84]. In the rotenone (Rot)-mediated (0.3 mg/kg) airway injury and allergic airway murine model, Miro1, a mitochondria Rho GTPase 1, is capable of facilitating mitochondria transfer from MSCs to damaged epithelial cells to reverse mitochondria dysfunction [85]. Altogether, MSC-EVs serve as important mediators which target on the immunomodulation and airway reconstruction, but their mechanisms of action are still under investigation.
Chronic Obstructive Pulmonary Disease (COPD)
COPD is a common, preventable, and treatable lung disease that is characterized by mucous hypersecretion and ciliary abnormalities, airflow obstruction and hyperinflation, gas change dysfunction and pulmonary hypertension, which damages airways (bronchitis-bronchiolitis) and alveoli (emphysema), leading to chronic inflammatory responses, persistent respiratory symptoms and airflow limitations [86, 87].
Cigarette smoke (CS) has been recognized as the foremost risk factor contributing to COPD development. The global strategy for the diagnosis, management, and prevention of COPD 2017 (GOLD2017) elucidated that COPD is projected to be the third leading cause of death worldwide by 2020 [88]. Current pharmacological agents are are poorly responsive to the disease’s progress and mortality [88]. Based on a growing body of evidence, it has been clear that MSCs have brought hopeful and promising effects in COPD. Shigemura et al were the first to establish that adipose tissue-derived stromal cells (ASCs) secrete HGF to repair pulmonary emphysema, and improve gas exchange and exercise tolerance [89]. Simultaneously, MSCs also modulate gene expression profiles of adjacent cells to reduce airway inflammation and restore alveolar architecture. In addition, Kim and colleagues have shown that compared to a control group, 834 genes were differentially expressed after human cord blood-derived-MSC administration in a smoke-induced COPD mouse lung model, more specifically, the genes (Hbb and Hba) with oxygen transport and antioxidant functions are significantly increased on day 1 and day 14 [90]. In an elastase-induced emphysema model, MSC administration facilitates the protease/anti-protease balance and decreases the activity of matrix metalloproteinase 9 [91]. Additionally, MSCs overexpressed with the all-trans retinoic acid (ATRA) combined with p-70S6 kinase-1 (p70S6k1) enhances the therapeutic effects in elevating static lung compliance, alveolar surface area, and decreases mean line intercepts [92]. A large amount of promising results contribute to MSC application in COPD patients. To the best of our knowledge, the first clinical trial (NCT00683722) was initiated by Weiss and colleagues, which is a Phase II, multicentre, randomized, double-blind placebo-controlled study, study involving sixty-two patients with GOLD stage II and III COPD patients. After a 2-year follow up, no infusional toxicities or serious adverse events related to MSC administration were deemed to have happened, and a significant decrease in C-reactive protein (CRP) level was observed in MSC-injected patients [93].
MSC-EVs have also exhibited protective effects in experimental COPD. Based on a chronic CS-induced COPD mice model, MSC-exosomes were shown to significantly improve lung function, including elevated O2 saturation, pH, PaO2, IL-10 secretion, and to decrease pro-inflammatory cytokine production (TNF-α, IL-12), the total number of lung-infiltrated macrophages, the capacities of antigen-presenting alveolar macrophages, IL-17A producing-NK/NKT cells, neutrophils to attenuate inflammation. Moreover, the exosomes are capable of affecting the migratory and antigen-presenting properties of DCs, which contribute to the attenuated activation of CD4+ and CD8+ T lymphocytes. Importantly, this study has also demonstrated that inhalation of MSC-exosomes indicate an improved FEV1, PEF, 6-min walking distance (6MWD) and quality of life in COPD patients, with alleviated emphysematous changes, including less hyperexpanded lung, less flattened diaphragms and reduced centrilobular and paraseptal emphysema [94]. In what could be another mechanism for MSC-EVs in COPD, Kim et al have shown that unlike MSC-derived natural exosomes, MSC-derived artificial nanovesicles (3 × 107 artificial nanovesicle particles generated from 7 × 107 ASCs) display a more efficient regenerative capacity to reduce the mean linear intercept (MLI) primarily through activating the FGF-2 signalling pathway [95]. In addition, functional mitochondria packaged in EVs have recapitulated MSC effects in preclinical models of COPD. iPSC-derived MSCs transfer their mitochondria to human bronchial epithelial cells through tunnelling nanotubes to alleviate CS-induced damage and to rescue the dysfunctional mitochondria of human airway smooth muscle cells in rats [9, 96]. Maremanda et al have demonstrated that MSC-exosomes also modify mitochondrial genes in bronchial epithelial cells, including enhancing fusion gene expression (mfn1, mfn2, and opa1) [10] (Fig. 2). To date, MSC-EVs, as a new frontier, have provided convincing evidence of positive effects in COPD by modulating chronic inflmammtion, inhibiting emphysema, and restoring dysfunctional mitochondria.
Role of MSC-EVs in COPD. MSC-EVs elevate O2 saturation, pH, PaO2, IL-10 secretion, and decrease pro-inflammatory cytokine production (TNF-α, IL-12). Functional mitochondria are donated by MSCs to bronchial epithelial cells through nanotubes, contributing to enhance oxidative phosphorylation of the targeted cells. MSC-EVs uptake by Alveolar type II cells stimulate FGF2 intracellular signalling activation. MSC-EVs also downregulate the pro-inflammatory cytokine secretion (TNF-α, IL-12), deactivate CD4+/CD8+ T cells, and suppress the migration and antigen presenting property of dendritic cells.
Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) is defined as a chronic, degenerative and progressive lung disease characterized by alveolar epithelial cell dysfunction, fibroblast proliferation, extracellular matrix collagen accumulation, and interstitial inflammation, leading to exertional dyspnoea, dry cough, weight loss malaise and arthralgia [97]. The comprehensive understanding of IPF pathogensis and effective treatments remains elusive. Available data have shown that MSC administration restores a bleomycin-induced lung injury model with a reduction in inflammatory response and collagen deposition, and an improved Ashcroft score [98, 99]. Reddy and colleagues have demonstrated that MSCs are capable of ameliorating the expression of pro-inflammatory (IL-1b, TNF-β, etc.), pro-fibrotic (bFGF, CTGF, etc) transcripts in injured lungs, and maintaining MMP-TIMP balance [100], correcting the inappropriate epithelial-mesenchymal relationships through stanniocalcin-1 to ameliorate oxidative stress and endoplasmic reticulum stress [101]. Moreover, Akram et al have reported that MSC-CM promotes human small airway epithelial cell (SAEC) wound repair by secreting an array of proteins (Fibronectin, Lumican, Periostin, IGFBP) [102], increasing the amount of Tregs, decreasing cytotoxic T cells coupled with a concomitant suppression in α-smooth muscle actin (α-SMA) [103], contributing to a reduced hydroxyproline (HYP) deposition, myeloid differentiation primary response gene 88 (MyD88), and TGF-β signaling activation [104]. Recently, Gad et al. have shown that the therapeutic anti-fibrotic properties of MSCs are mediated through the inhibition of SMAD-3/TGF-β signalling [105].
Unfortunately, research into MSC-EV effects on IPF is limited. Mansouri and colleagues have presented that MSC-EVs are able to reduce the degree of whole lung apoptosis, reverting pulmonary fibrosis, improving the Ashcroft score, and increasing the number of alveolar macrophages (CD206) and nonclassical monocytes. Additionally, bioinformatics analysis has revealed that eighty-four peptides varied significantly between MSC-EV treatment and fibroblast-derived EV treatment with myeloid/monocyte cells [106]. More recently, Wan et al have conducted research on MSC-EV effects on pulmonary fibroblasts, and have demonstrated that the EVs suppress fibroblast proliferation, migration, invasion, and differentiation in IPF, confirmed by Cell Counting Kit (CCK-8), Transwell assay, and gain- and loss-of-function assays through overexpressing miR-29-3p [107].
Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH) is a chronic and devastating disease in which extensive obliterative changes are associated with elevated pulmonary arteries pressure, pulmonary vascular resistance, and right ventricular (RV) dysfunction, resulting in vascular fibrosis and stiffening [108, 109]. Mounting evidence has shown that treatment with rat MSC or human MSC treatment is able to decrease pulmonary vascular resistance, improve vascular endothelial function and right ventricular function in the monocrotaline or Su5416/hypoxia-injured lung [110,111,112,113] through regulating [Ca2+]i signal-associated cellular behaviours [114], normalizing the expression levels of apoptosis (active-caspase-3), cellular proliferation (p-38 MAPK and ERK5), and inflammation markers (TNF-α, IL-1β, IL-6) [115], suppressing TLR-4 signalling [116], expressing Heme Oxygenase-1 (HO-1), enhancing let-7a expression [117], and dampening endothelial-mesenchymal transition (EndMT) [118, 119]. Moreover, high throughput sequencing has demonstrated that six miRNAs of MSCs (upregulated: miR573 and miR1246; downregulated: miR206, miR-133a-3p, miR-141-3p and miR-200a-3p) are differentially expressed with co-culturing with human pulmonary arterial endothelial cells (HPAECs) [120]. Simultaneously, there have been several clinical trials completed aiming at the MSCs effects on IPF patients. Tzouvelekis and colleagues conducted a prospective, non-randomized, no placebo-controlled, phase 1b clinical trial (EHD33/1SC/16-02-2010) to investigate the safety of MSCs in IPF, which showed that MSC administration is an acceptable safe treatment regimen to IPF. However, this study did not deteriorate in functional parameters and indicators of quality of life [121]. Similarly, the other dose-escalation phase 1b trial (NCT01385644) has indicated there is no significant change in forced ventilation capacity (FVC), diffusing capacity of the lungs for carbon monoxide (DLCO), 6MWD and CT fibrosis score of the IPF patients compared with baseline after 6 months [122]. Nevertheless, the AETHER trial (NCT02013700), the allogeneic human MSCs in patients with IPF via intravenous delivery, has demonstrated a 3.0% decline in % predicted FVC and 5.4% mean decline in % predicted DLCO. More recently, the first-in-human high-cumulative-dose stem cell therapy in IPF patients was conducted by Averyanov and colleagues. Twenty patients with a current FVC ≥ 40% of predicted and DLCO ≥20% with a lung function decline (FVC and DLCO) ≥10% over the last 12 months were recruited into a phase I/IIa study, and received two intravenous doses of MSCs (2*108 cells) every three months (total amount: 1.6*109 cells). After the study was completed, no significant adverse effects were found in the MSC-administrated group, and they were observed having a better outcome for the 6MWD, for DLCO in 26 weeks, and for FVC in 39 weeks compared with the placebo group [123].
Intravenous injection of MSC-EVs in the monocrotaline-induced PH (MCT-PH) rat model has indicated similar effects to those of MSCs in ameliorating the mean pulmonary artery pressure (mPAP), mean right ventricle pressure (mRVP), RV hypertrophy, the pulmonary arteriole area index (AI) and the thickness index (PI) [124], enhancing macrophage polarization [125], and deactivating EndMT [126]. According to the research investigating paracrine mechanisms, MSC-EVs are capable of suppressing the signal transducer and activator of transcription 3 (STAT3), and upregulating the miR17 superfamily and miR204 in the context of PAH rats [127]. In addition, MSC-exosome packaged miR191 restores monocrotaline pyrrole (MCTP)-induced lung injury by repressing bone morphogenetic protein receptor 2 (BMPR2) [128]. Moreover, microvesicles derived from MSCs promote ACE2 mRNA and plasma levels of Ang- (1–7) in the injured lung [129], and MSC-exosome administration increases the expression of pyruvate dehydrogenase (PDH) and glutamate dehydrogenase 1 (GLUD1), leading to improved mitochondrial health in the hypoxia-induced PAH mouse model [130]. However, even though many promising data support MSC therapeutic effects for PH, to date, no clinical trials in this area can be found in “ClincalTrials.gov”.
Silicosis
Silicosis is a preventable but chronic, progressive, and fatal occupational respiratory disease caused by the long-term inhalation of respirable crystalline silica dust, which lacks specific pharmacological treatment and potentially increases the morbidity of pulmonary tuberculosis [131]. Mounting evidence has shown that MSC transplantation contributes to a remissive effect on silica-induced lung fibrosis [132, 133] through decreasing the level of Caspase-3 protein [134], downregulating the expressions of fibrosis marker proteins (Vimentin and α-smooth actin) [135], the mRNA levels of collagen I, collagen III, and fibronectin, and the secretion of TGF-β and hydroxyproline [136], in parallel with elevating the ratio of Bcl-2/Bax, the expression of epithelial marker protein (E-cadherin, cytokeratin19). Due to the low prevalence rate, only one clinical trial (NCT01977131) was found to assess the MSC effect in patients with pulmonary silicosis. Autologous BM-MSCs transfected with human HGF cDNA (MSCs/HGF) were administrated intravenously at a dose of 2*106/kg in four patients. It has been found that MSCs/HGF were capable of ameliorating the symptoms of cough and chest distress, and improving pulmonary function. Moreover, in the labtorary tests of peripheral blood from the patients, the ratio of the peripheral CD4+/CD8+ was increased, and serum IgG levels were decreased [137].
MSC-EVs have also shown a therapeutic effect on silica-induced experimental silicosis as a cell replacement of MSCs. Bandeira et al have stated that MSC-EVs lead to a reduction in collagen fiber content, lung static elastance, size of granuloma, and the number of macrophages inside granuloma and in the alveolar sept in the silica intratracheal instillation of C57BL/6 mice [138]. Moreover, intravenous administration of human MSC-exosomes is capable of reducing the extent of Ly6Chi monocyte infiltration into the injured lung, the size of silicotic nodules, the total number of white cells in BALF, and the expression of inflammatory (TNF-α, IL-6) and pro-fibrotic genes (COL1A1) in the lung tissue [139]. However, Choi et al have shown that even though MSC-microvesicles present beneficial effects to silica-mediated silicosis, their therapeutic efficiency is less than that of MSC transplantation [140]. Finally, it has become clear that MSCs or MSC-EVs have shown therapeutic potential to pulmonary silicosis, but dissecting the compensive biological mechanisms is the next milestone for the application in clinicial work.
Challenges in MSC-EV Application in Clinics
Despite the wealth of promising preclinical results for MSC-EV application in lung diseases, unfortunately, until now there have been only two clinical trials in regard to evaluating MSC-EV effects on SARS-CoV2-induced severe pneumonia and BPD. To date, there have been no large, randomized, and placebo-controlled clinical trials aimed at assessing the effect of MSC-EVs on lung diseases due to a limited understanding of the molecular mechanisms involved. As a cell-free alternative therapeutic agent, research into MSC-EV remains in its infancy and many questions need to have definitive answers. Among the most urgent questions are: 1) which linage or origin is the best to isolate the EVs? 2) which systemic (intravenous, intraperitoneal) or local (intratracheal, intrabronchial, intrapleural, intranasal) routes are suitable for various kinds of lung diseases? 3) Of single or multiple administration, which is superior? 4) Does MSC-EV therapy play a protagonist or adjuvant role in different lung diseases? 5) Which quali-quantitative compositions play key roles in the biological effects of MSC-EVs? 6) What are the gold-standard animal models for human lung diseases to confirm the MSC effects? For instance, in research into the effect of MSC-EVs on IPF, bleomycin-induced lung injury is mostly chosen to mimic the IPF microenvironment. This is regarded as the animal model which is most akin to the human pathophysiology of IPF, but the processes that drive IPF are complex, and bleomycin-induced lung injury is widely used in pulmonary fibrosis without considering its pathogenesis.
The other challenge of MSC-EV application in lung diseases for rapid translation from bench to bedside is the absence of standardized and consolidated criteria of EV production and separation. The differences in EV isolation and characterization retain a great deal of heterogeneous features that are at odds with the homogeneity required for the clinic. The members of four academic societies (ISCT, ISCT, ISBT, and SOCRATES) have identified the key defining physical and biological characteristics of MSC-sEVs in a position paper, but it did not mention how to predict the therapeutic potency of MSC-EVs within quantifiable and reproducible parameters [141]. Obtaining a deep understanding of MSC-sEV biology and developing an appropriate functional assay to test its therapeutic properties will facilitate the development of MSC-sEVs as an off-the-shelf alternative treatment for lung diseases.
What still remains a mystery is the appropriate therapeutic doses for each lung disease. Due to the lack of standard protocols of EV isolation and characterization, studies from different institutes are using various methods to quantify MSC-EVs. Protein concentration or particle amount of MSC-EVs are widely used to illustrate the dose of MSC-EVs in a large number of publications. The lack of standard protocols contributes to the doubtful and confusing results for clinicians if they plan to design clinical trials. Furthermore, the optimal dose for the phenotypes of lung diseases is also largely unknown. For example, ARDS subphenotypes, comprising hypo-inflammatory and hyper-inflammatory phenotypes, have been identified by Calfee et al [142], but which subphenotype is more suitable for MSC-EV treatment remains uncertain.
Finally, further investigations into how to scale up and develop the specific protocol of good manufacturing practice (GMP) for MSC-EV production are required as they are needed in large quantities based on the experimental respiratory animal model. Furthermore, it has been identified that the senescence of BM-MSCs, ADSCs or UC-MSCs may limit their use for isolating large-scale MSC-EVs, but embryonic stem cell-derived MSCs have shown the ability to produce large amounts of MSC-EVs with no changed in quantity and quality [143, 144]. Nevertheless, even though these publications provide some instructions for the biomanufacturing of MSC-EVs [145, 146], the field of large-scale EV biomanufacturing schemes remains unexplored.
Future Perspectives
Respiratory diseases still threaten millions of people in all regions of the world and the strategies for their prevention and control are urgently required. More public attention and research funding should be given to respiratory diseases due to the increasing population and deteriorating environment. The findings concerning MSC-EV effects on pulmonary regeneration are consistent, and their potent capacities in immunomodulation are evident. The fact that MSC-EVs show less potential for immunogenicity and tumorigenicity contributes to the possibility of their clinical application in lung diseases, and the beneficial effects of their targeting immunoregulation and tissue repair result in the possibility of numerous biological advantages in the treatment of acute and chronic lung injury. Accordingly, substantial work in dissecting the exact molecular mechanisms of MSC-EV effects is required by further investigation. Otherwise, MSC-based cell-free alternative therapeutic regimens will remain imprecise and speculative.
References
Forum of International Respiratory Societies. (2017). The Global Impact of Respiratory Disease – (2nd ed.). Sheffi eld: European Respiratory Society.
Labaki, W. W., & Han, M. L. K. (2020). Chronic respiratory diseases: A global view. The Lancet Respiratory Medicine, 8, 531–533.
Soriano, J. B., et al. (2020). Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the global burden of Disease study 2017. The Lancet Respiratory Medicine, 8, 585–596.
Matthay, M. A., Zemans, R. L., Zimmerman, G. A., et al. (2019). Acute respiratory distress syndrome. Nature Reviews Disease Primers, 5, 18. https://doi.org/10.1038/s41572-019-0069-0.
Burnham, E. L., Janssen, W. J., Riches, D. W. H., Moss, M., & Downey, G. P. (2014). The fibroproliferative response in acute respiratory distress syndrome: Mechanisms and clinical significance. The European Respiratory Journal, 43, 276–285.
Harrell, C. R., Sadikot, R., Pascual, J., Fellabaum, C., Jankovic, M. G., Jovicic, N., Djonov, V., Arsenijevic, N., & Volarevic, V. (2019). Mesenchymal stem cell-based therapy of inflammatory lung diseases: Current understanding and future perspectives. Stem Cells International, 2019, 1–14.
Schrepfer, S., et al. (2007). Stem cell transplantation: The lung barrier. Transplantation Proceedings, 39, 573–576.
Porzionato, A., Zaramella, P., Dedja, A., Guidolin, D., van Wemmel, K., Macchi, V., Jurga, M., Perilongo, G., de Caro, R., Baraldi, E., & Muraca, M. (2019). Intratracheal administration of clinical-grade mesenchymal stem cell-derived extracellular vesicles reduces lung injury in a rat model of bronchopulmonary dysplasia. Am. J. Physiol. - Lung Cell. Mol. Physiol., 316, L6–L19.
Li, X., Zhang, Y., Yeung, S. C., Liang, Y., Liang, X., Ding, Y., Ip, M. S., Tse, H. F., Mak, J. C., & Lian, Q. (2014). Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. American Journal of Respiratory Cell and Molecular Biology, 51, 455–465.
Maremanda, K. P., Sundar, I. K., & Rahman, I. (2019). Protective role of mesenchymal stem cells and mesenchymal stem cell-derived exosomes in cigarette smoke-induced mitochondrial dysfunction in mice. Toxicology and Applied Pharmacology, 385, 114788.
Konala, V. B. R., Mamidi, M. K., Bhonde, R., Das, A. K., Pochampally, R., & Pal, R. (2016). The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy, 18, 13–24.
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., Antoniou, A., Arab, T., Archer, F., Atkin-Smith, G. K., Ayre, D. C., Bach, J. M., Bachurski, D., Baharvand, H., Balaj, L., Baldacchino, S., Bauer, N. N., Baxter, A. A., Bebawy, M., Beckham, C., Bedina Zavec, A., Benmoussa, A., Berardi, A. C., Bergese, P., Bielska, E., Blenkiron, C., Bobis-Wozowicz, S., Boilard, E., Boireau, W., Bongiovanni, A., Borràs, F. E., Bosch, S., Boulanger, C. M., Breakefield, X., Breglio, A. M., Brennan, M. Á., Brigstock, D. R., Brisson, A., Broekman, M. L. D., Bromberg, J. F., Bryl-Górecka, P., Buch, S., Buck, A. H., Burger, D., Busatto, S., Buschmann, D., Bussolati, B., Buzás, E. I., Byrd, J. B., Camussi, G., Carter, D. R. F., Caruso, S., Chamley, L. W., Chang, Y. T., Chen, C., Chen, S., Cheng, L., Chin, A. R., Clayton, A., Clerici, S. P., Cocks, A., Cocucci, E., Coffey, R. J., Cordeiro-da-Silva, A., Couch, Y., Coumans, F. A. W., Coyle, B., Crescitelli, R., Criado, M. F., D’Souza-Schorey, C., Das, S., Datta Chaudhuri, A., de Candia, P., de Santana Junior, E. F., de Wever, O., del Portillo, H. A., Demaret, T., Deville, S., Devitt, A., Dhondt, B., di Vizio, D., Dieterich, L. C., Dolo, V., Dominguez Rubio, A. P., Dominici, M., Dourado, M. R., Driedonks, T. A. P., Duarte, F. V., Duncan, H. M., Eichenberger, R. M., Ekström, K., el Andaloussi, S., Elie-Caille, C., Erdbrügger, U., Falcón-Pérez, J. M., Fatima, F., Fish, J. E., Flores-Bellver, M., Försönits, A., Frelet-Barrand, A., Fricke, F., Fuhrmann, G., Gabrielsson, S., Gámez-Valero, A., Gardiner, C., Gärtner, K., Gaudin, R., Gho, Y. S., Giebel, B., Gilbert, C., Gimona, M., Giusti, I., Goberdhan, D. C. I., Görgens, A., Gorski, S. M., Greening, D. W., Gross, J. C., Gualerzi, A., Gupta, G. N., Gustafson, D., Handberg, A., Haraszti, R. A., Harrison, P., Hegyesi, H., Hendrix, A., Hill, A. F., Hochberg, F. H., Hoffmann, K. F., Holder, B., Holthofer, H., Hosseinkhani, B., Hu, G., Huang, Y., Huber, V., Hunt, S., Ibrahim, A. G. E., Ikezu, T., Inal, J. M., Isin, M., Ivanova, A., Jackson, H. K., Jacobsen, S., Jay, S. M., Jayachandran, M., Jenster, G., Jiang, L., Johnson, S. M., Jones, J. C., Jong, A., Jovanovic-Talisman, T., Jung, S., Kalluri, R., Kano, S. I., Kaur, S., Kawamura, Y., Keller, E. T., Khamari, D., Khomyakova, E., Khvorova, A., Kierulf, P., Kim, K. P., Kislinger, T., Klingeborn, M., Klinke II, D. J., Kornek, M., Kosanović, M. M., Kovács, Á. F., Krämer-Albers, E. M., Krasemann, S., Krause, M., Kurochkin, I. V., Kusuma, G. D., Kuypers, S., Laitinen, S., Langevin, S. M., Languino, L. R., Lannigan, J., Lässer, C., Laurent, L. C., Lavieu, G., Lázaro-Ibáñez, E., le Lay, S., Lee, M. S., Lee, Y. X. F., Lemos, D. S., Lenassi, M., Leszczynska, A., Li, I. T. S., Liao, K., Libregts, S. F., Ligeti, E., Lim, R., Lim, S. K., Linē, A., Linnemannstöns, K., Llorente, A., Lombard, C. A., Lorenowicz, M. J., Lörincz, Á. M., Lötvall, J., Lovett, J., Lowry, M. C., Loyer, X., Lu, Q., Lukomska, B., Lunavat, T. R., Maas, S. L. N., Malhi, H., Marcilla, A., Mariani, J., Mariscal, J., Martens-Uzunova, E. S., Martin-Jaular, L., Martinez, M. C., Martins, V. R., Mathieu, M., Mathivanan, S., Maugeri, M., McGinnis, L. K., McVey, M. J., Meckes Jr., D. G., Meehan, K. L., Mertens, I., Minciacchi, V. R., Möller, A., Møller Jørgensen, M., Morales-Kastresana, A., Morhayim, J., Mullier, F., Muraca, M., Musante, L., Mussack, V., Muth, D. C., Myburgh, K. H., Najrana, T., Nawaz, M., Nazarenko, I., Nejsum, P., Neri, C., Neri, T., Nieuwland, R., Nimrichter, L., Nolan, J. P., Nolte-’t Hoen, E. N. M., Noren Hooten, N., O’Driscoll, L., O’Grady, T., O’Loghlen, A., Ochiya, T., Olivier, M., Ortiz, A., Ortiz, L. A., Osteikoetxea, X., Østergaard, O., Ostrowski, M., Park, J., Pegtel, D. M., Peinado, H., Perut, F., Pfaffl, M. W., Phinney, D. G., Pieters, B. C. H., Pink, R. C., Pisetsky, D. S., Pogge von Strandmann, E., Polakovicova, I., Poon, I. K. H., Powell, B. H., Prada, I., Pulliam, L., Quesenberry, P., Radeghieri, A., Raffai, R. L., Raimondo, S., Rak, J., Ramirez, M. I., Raposo, G., Rayyan, M. S., Regev-Rudzki, N., Ricklefs, F. L., Robbins, P. D., Roberts, D. D., Rodrigues, S. C., Rohde, E., Rome, S., Rouschop, K. M. A., Rughetti, A., Russell, A. E., Saá, P., Sahoo, S., Salas-Huenuleo, E., Sánchez, C., Saugstad, J. A., Saul, M. J., Schiffelers, R. M., Schneider, R., Schøyen, T. H., Scott, A., Shahaj, E., Sharma, S., Shatnyeva, O., Shekari, F., Shelke, G. V., Shetty, A. K., Shiba, K., Siljander, P. R. M., Silva, A. M., Skowronek, A., Snyder II, O. L., Soares, R. P., Sódar, B. W., Soekmadji, C., Sotillo, J., Stahl, P. D., Stoorvogel, W., Stott, S. L., Strasser, E. F., Swift, S., Tahara, H., Tewari, M., Timms, K., Tiwari, S., Tixeira, R., Tkach, M., Toh, W. S., Tomasini, R., Torrecilhas, A. C., Tosar, J. P., Toxavidis, V., Urbanelli, L., Vader, P., van Balkom, B. W. M., van der Grein, S. G., van Deun, J., van Herwijnen, M. J. C., van Keuren-Jensen, K., van Niel, G., van Royen, M. E., van Wijnen, A. J., Vasconcelos, M. H., Vechetti Jr., I. J., Veit, T. D., Vella, L. J., Velot, É., Verweij, F. J., Vestad, B., Viñas, J. L., Visnovitz, T., Vukman, K. V., Wahlgren, J., Watson, D. C., Wauben, M. H. M., Weaver, A., Webber, J. P., Weber, V., Wehman, A. M., Weiss, D. J., Welsh, J. A., Wendt, S., Wheelock, A. M., Wiener, Z., Witte, L., Wolfram, J., Xagorari, A., Xander, P., Xu, J., Yan, X., Yáñez-Mó, M., Yin, H., Yuana, Y., Zappulli, V., Zarubova, J., Žėkas, V., Zhang, J. Y., Zhao, Z., Zheng, L., Zheutlin, A. R., Zickler, A. M., Zimmermann, P., Zivkovic, A. M., Zocco, D., & Zuba-Surma, E. K. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7,1. https://doi.org/10.1080/20013078.2018.1535750.
Todorova, D., Simoncini, S., Lacroix, R., Sabatier, F., & Dignat-George, F. (2017). Extracellular vesicles in angiogenesis. Circulation Research, 120, 1658–1673.
Tetta, C., Deregibus, M. C., & Camussi, G. (2020). Stem cells and stem cell-derived extracellular vesicles in acute and chronic kidney diseases: Mechanisms of repair. Ann. Transl. Med., 8, 570–570.
Raposo, G., & Stoorvogel, W. (2013). Extracellular vesicles: Exosomes, microvesicles, and friends. Journal of Cell Biology, 200, 373–383. https://doi.org/10.1083/jcb.201211138.
Van Niel, G., D’Angelo, G., & Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology, 19, 213–228. https://doi.org/10.1038/nrm.2017.125.
Quesenberry, P. J., Goldberg, L. R., Aliotta, J. M., Dooner, M. S., Pereira, M. G., Wen, S., & Camussi, G. (2014). Cellular phenotype and extracellular vesicles: Basic and clinical considerations. Stem Cells and Development, 23, 1429–1436. https://doi.org/10.1089/scd.2013.0594.
Camussi, G., Deregibus, M. C., & Cantaluppi, V. (2013). Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochemical Society Transactions, 41, 283–287. https://doi.org/10.1042/BST20120192.
Zemans, R. L., & Matthay, M. A. (2017). What drives neutrophils to the alveoli in ARDS? Thorax, 72, 1–3.
Baek, G., Choi, H., Kim, Y., Lee, H. C., & Choi, C. (2019). Mesenchymal stem cell-derived extracellular vesicles as therapeutics and as a drug delivery platform. Stem Cells Translational Medicine, 8, 880–886.
Abraham, A., & Krasnodembskaya, A. (2020). Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Translational Medicine, 9, 28–38.
Biancone, L., Bruno, S., Deregibus, M. C., Tetta, C., & Camussi, G. (2012). Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrology, Dialysis, Transplantation, 27, 3037–3042. https://doi.org/10.1093/ndt/gfs168.
Collino, F., Deregibus, M. C., Bruno, S., Sterpone, L., Aghemo, G., Viltono, L., Tetta, C., & Camussi, G. (2010). Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One, 5. https://doi.org/10.1371/journal.pone.0011803.
Bruno, S., Chiabotto, G., Favaro, E., Deregibus, M. C., & Camussi, G. (2019). Role of extracellular vesicles in stem cell biology. American Journal of Physiology. Cell Physiology, 317, C303–C313.
Davidson, L., & Berkelhamer, S. (2017). Bronchopulmonary dysplasia: Chronic lung Disease of infancy and long-term pulmonary outcomes. Journal of Clinical Medicine, 6, 4.
Coalson, J. J. (2006). Pathology of Bronchopulmonary dysplasia. Seminars in Perinatology, 30, 179–184. https://doi.org/10.1053/j.semperi.2006.05.004.
Nardiello, C., Mižíková, I., & Morty, R. E. (2017). Looking ahead: Where to next for animal models of bronchopulmonary dysplasia? Cell and Tissue Research, 367, 457–468. https://doi.org/10.1007/s00441-016-2534-3.
Moreira, A., Winter, C., Joy, J., Winter, L., Jones, M., Noronha, M., Porter, M., Quim, K., Corral, A., Alayli, Y., Seno, T., Mustafa, S., Hornsby, P., & Ahuja, S. (2020). Intranasal delivery of human umbilical cord Wharton’s jelly mesenchymal stromal cells restores lung alveolarization and vascularization in experimental bronchopulmonary dysplasia. Stem Cells Translational Medicine, 9, 221–234.
Kwon, J. H., Kim, M., Bae, Y. K., Kim, G. H., Choi, S. J., Oh, W., Um, S., & Jin, H. J. (2019). Decorin secreted by human umbilical cord blood-derived mesenchymal stem cells induces macrophage polarization via CD44 to repair hyperoxic lung injury. International Journal of Molecular Sciences, 20.
Yue, Y., Luo, Z., Liao, Z., Zhang, L., Liu, S., Wang, M., Zhao, F., Cao, C., Ding, Y., & Yue, S. (2019). Excessive activation of NMDA receptor inhibits the protective effect of endogenous bone marrow mesenchymal stem cells on promoting alveolarization in bronchopulmonary dysplasia. Am. J. Physiol. - Cell Physiol., 316, C815–C827.
Chen, C. M., & Chou, H. C. (2018). Human mesenchymal stem cells attenuate hyperoxia-induced lung injury through inhibition of the renin-angiotensin system in newborn rats. American Journal of Translational Research, 10, 2628–2635.
Chen, C. M., Lin, W., Huang, L. T., & Chou, H. C. (2017). Human mesenchymal stem cells ameliorate experimental pulmonary hypertension induced by maternal inflammation and neonatal hyperoxia in rats. Oncotarget, 8, 82366–82375.
Chou, H. C., Li, Y. T., & Chen, C. M. (2016). Human mesenchymal stem cells attenuate experimental bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. American Journal of Translational Research, 8, 342–353.
Li, Z., Gong, X., Li, D., Yang, X., Shi, Q., & Ju, X. (2020). Intratracheal transplantation of amnion-derived Mesenchymal stem cells ameliorates Hyperoxia-induced neonatal Hyperoxic lung injury via Aminoacyl-peptide hydrolase. Int. J. Stem Cells, 13, 221–236.
Kim, M., Kwon, J. H., Bae, Y. K., Kim, G. H., Um, S., Ha, J., Choi, S. J., Oh, W., & Jin, H. J. (2020). Soluble PTX3 of human umbilical cord blood-derived Mesenchymal stem cells attenuates Hyperoxic lung injury by activating macrophage polarization in neonatal rat model. Stem Cells International, 2020, 1–18.
Reiter, J., et al. (2017). Stromal derived factor-1 mediates the lung regenerative effects of mesenchymal stem cells in a rodent model of bronchopulmonary dysplasia. Respiratory Research, 18, 1–11.
Aslam, M., Baveja, R., Liang, O. D., Fernandez-Gonzalez, A., Lee, C., Mitsialis, S. A., & Kourembanas, S. (2009). Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. American Journal of Respiratory and Critical Care Medicine, 180, 1122–1130.
Suzuki, T., Sato, Y., Yamamoto, H., Kato, T., Kitase, Y., Ueda, K., Mimatsu, H., Sugiyama, Y., Onoda, A., Saito, S., Takahashi, Y., Nakayama, T., & Hayakawa, M. (2020). Mesenchymal stem/stromal cells stably transduced with an inhibitor of CC chemokine ligand 2 ameliorate bronchopulmonary dysplasia and pulmonary hypertension. Cytotherapy, 22, 180–192.
Chang, Y. S., et al. (2014). Mesenchymal Stem Cells for Bronchopulmonary Dysplasia: Phase 1 Dose-Escalation Clinical Trial. J. Pediatr, 164, 966–972.e6.
Willis, G. R., Fernandez-Gonzalez, A., Anastas, J., Vitali, S. H., Liu, X., Ericsson, M., Kwong, A., Mitsialis, S. A., & Kourembanas, S. (2018). Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. American Journal of Respiratory and Critical Care Medicine, 197, 104–116.
Braun, R. K., Chetty, C., Balasubramaniam, V., Centanni, R., Haraldsdottir, K., Hematti, P., & Eldridge, M. W. (2018). Intraperitoneal injection of MSC-derived exosomes prevent experimental bronchopulmonary dysplasia. Biochemical and Biophysical Research Communications, 503, 2653–2658.
Chaubey, S., et al. (2018). Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Research & Therapy, 9, 1–26.
Thompson, B. T., Chambers, R. C., & Liu, K. D. (2017). Acute respiratory distress syndrome. The New England Journal of Medicine, 377, 562–572.
Fang, X., Neyrinck, A. P., Matthay, M. A., & Lee, J. W. (2010). Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. The Journal of Biological Chemistry, 285, 26211–26222.
Ortiz, L. A., DuTreil, M., Fattman, C., Pandey, A. C., Torres, G., Go, K., & Phinney, D. G. (2007). Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proceedings of the National Academy of Sciences of the United States of America, 104, 11002–11007.
Hu, S., et al. (2016). The hepatocyte growth factor-expressing character is required for mesenchymal stem cells to protect the lung injured by lipopolysaccharide in vivo. Stem Cell Research & Therapy, 7, 1–13.
Goolaerts, A., Pellan-Randrianarison, N., Larghero, J., et al. (2014). Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. American Journal of Physiology. Lung Cellular and Molecular Physiology, 306(11), L975–L985. https://doi.org/10.1152/ajplung.00242.2013.
Fang, X., et al. (2015). Human Mesenchymal stem (stromal) cells promote the resolution of acute lung injury in part through Lipoxin a 4. Journal of Immunology, 195, 875–881.
McAuley, D. F., et al. (2014). Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am. J. Physiol. - Lung Cell. Mol. Physiol., 306, 809–815.
Lee, J. W., Fang, X., Gupta, N., Serikov, V., & Matthay, M. A. (2009). Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. U. S. A., 106, 16357–16362.
Wilson, J. G., Liu, K. D., Zhuo, H., Caballero, L., McMillan, M., Fang, X., Cosgrove, K., Vojnik, R., Calfee, C. S., Lee, J. W., Rogers, A. J., Levitt, J., Wiener-Kronish, J., Bajwa, E. K., Leavitt, A., McKenna, D., Thompson, B. T., & Matthay, M. A. (2015). Phase 1 clinical trial designs phase 1 designs. The Lancet Respiratory Medicine, 3, 24–32.
Bellingan G, Jacono F, Bannard-Smith J, Brealey D, Meyer N, Thickett D, et al. (2019). Ting, primary analysis of a phase 1/2 study to assess MultiStem® cell therapy, a regenerative Advanced Therapy Medicinal Product (ATMP), in Acute Respiratory Distress Syndrome (MUST-ARDS). B14. Late Break Clin Trials, 199, A7353. https://doi.org/10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A7353.
Leng, Z., Zhu, R., Hou, W., Feng, Y., Yang, Y., Han, Q., Shan, G., Meng, F., du, D., Wang, S., Fan, J., Wang, W., Deng, L., Shi, H., Li, H., Hu, Z., Zhang, F., Gao, J., Liu, H., Li, X., Zhao, Y., Yin, K., He, X., Gao, Z., Wang, Y., Yang, B., Jin, R., Stambler, I., Lim, L. W., Su, H., Moskalev, A., Cano, A., Chakrabarti, S., Min, K. J., Ellison-Hughes, G., Caruso, C., Jin, K., & Zhao, R. C. (2020). Transplantation of ACE2- Mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging and Disease, 11, 216–228.
Liang, B. et al. (2020) Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. ChinaXiv. https://doi.org/10.3969/j.issn.2095-4344.2012.49.011.
Khatri, M., Richardson, L. A., & Meulia, T. (2018). Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Research & Therapy, 9, 1–13.
Monsel, A., Zhu, Y. G., Gennai, S., Hao, Q., Hu, S., Rouby, J. J., Rosenzwajg, M., Matthay, M. A., & Lee, J. W. (2015). Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. American Journal of Respiratory and Critical Care Medicine, 192, 324–336. https://doi.org/10.1164/rccm.201410-1765OC.
Li, J. W., Wei, L., Han, Z., & Chen, Z. (2019). Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur. J. Pharmacol, 852, 68–76.
Yi, X., Wei, X., Lv, H., An, Y., Li, L., Lu, P., Yang, Y., Zhang, Q., Yi, H., & Chen, G. (2019). Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Experimental Cell Research, 383, 111454. https://doi.org/10.1016/j.yexcr.2019.05.035.
Chen, W. X., et al. (2020). Microvesicles derived from human Wharton’s jelly mesenchymal stem cells enhance autophagy and ameliorate acute lung injury via delivery of miR-100. Stem Cell Research & Therapy, 11, 1–13.
Hao, Q., Gudapati, V., Monsel, A., Park, J. H., Hu, S., Kato, H., Lee, J. H., Zhou, L., He, H., & Lee, J. W. (2019). Mesenchymal stem cell–derived extracellular vesicles decrease lung injury in mice. Journal of Immunology, 203, 1961–1972.
Song, Y., Dou, H., Li, X., Zhao, X., Li, Y., Liu, D., Ji, J., Liu, F., Ding, L., Ni, Y., & Hou, Y. (2017). Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed Mesenchymal stem cells against Sepsis. Stem Cells, 35, 1208–1221. https://doi.org/10.1002/stem.2564.
Wang, J. et al. (2020) Mesenchymal Stem Cell–Derived Extracellular Vesicles Alleviate Acute Lung Injury Via Transfer of miR-27a-3p. Critical Care Medicine. 1 https://doi.org/10.1097/ccm.0000000000004315.
Zhu, Y. G., Feng, X. M., Abbott, J., Fang, X. H., Hao, Q., Monsel, A., Qu, J. M., Matthay, M. A., & Lee, J. W. (2014). Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells, 32, 116–125. https://doi.org/10.1002/stem.1504.
Tang, X. D., Shi, L., Monsel, A., Li, X. Y., Zhu, H. L., Zhu, Y. G., & Qu, J. M. (2017). Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by Ang-1 mRNA. Stem Cells, 35, 1849–1859. https://doi.org/10.1002/stem.2619.
Gennai, S., Monsel, A., Hao, Q., Park, J., Matthay, M. A., & Lee, J. W. (2015). Microvesicles derived from human Mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. American Journal of Transplantation, 15, 2404–2412. https://doi.org/10.1111/ajt.13271.
Park, J., Kim, S., Lim, H., Liu, A., Hu, S., Lee, J. H., Zhuo, H., Hao, Q., Matthay, M. A., & Lee, J. W. (2019). Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax, 74, 43–50.
Morrison, T. J., Jackson, M. V., Cunningham, E. K., Kissenpfennig, A., McAuley, D. F., O’Kane, C. M., & Krasnodembskaya, A. D. (2017). Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. American Journal of Respiratory and Critical Care Medicine, 196, 1275–1286.
Fergie, N., Todd, N., McClements, L., McAuley, D., O'Kane, C., & Krasnodembskaya, A. (2019). Hypercapnic acidosis induces mitochondrial dysfunction and impairs the ability of mesenchymal stem cells to promote distal lung epithelial repair. The FASEB Journal, 33, 5585–5598.
Papi, A., Brightling, C., Pedersen, S. E., & Reddel, H. K. (2018). Asthma. Lancet, 391, 783–800.
Boldrini-Leite, L. M., Michelotto, P. V., de Moura, S. A. B., et al. (2020). Lung tissue damage associated with allergic asthma in BALB/c mice could be controlled with a single injection of mesenchymal stem cells from human bone marrow up to 14 d after transplantation. Cell Transplantation. https://doi.org/10.1177/0963689720913254.
Boldrini-Leite, L. M., et al. (2020). Lung tissue damage associated with allergic asthma in BALB/c mice could be controlled with a single injection of Mesenchymal stem cells from human bone marrow up to 14 d after transplantation. Cell Transplantation, 29, 1–13.
Abreu, S. C., Antunes, M. A., Xisto, D. G., Cruz, F. F., Branco, V. C., Bandeira, E., Zola Kitoko, J., de Araújo, A. F., Dellatorre-Texeira, L., Olsen, P. C., Weiss, D. J., Diaz, B. L., Morales, M. M., & Rocco, P. R. M. (2017). Bone marrow, adipose, and lung tissue-derived murine mesenchymal stromal cells release different mediators and differentially affect airway and lung parenchyma in experimental asthma. Stem Cells Translational Medicine, 6, 1557–1567.
Keyhanmanesh, R., Rahbarghazi, R., & Ahmadi, M. (2018). Systemic transplantation of Mesenchymal stem cells modulates endothelial cell adhesion molecules induced by ovalbumin in rat model of asthma. Inflammation, 41, 2236–2245.
Song, X., Xie, S., Lu, K., & Wang, C. (2015). Mesenchymal stem cells alleviate experimental asthma by inducing polarization of alveolar macrophages. Inflammation, 38, 485–492.
Firinci, F., et al. (2011). Mesenchymal stem cells ameliorate the histopathological changes in a murine model of chronic asthma. International Immunopharmacology, 11, 1120–1126.
Royce, S. G., Mao, W., Lim, R., Kelly, K., & Samuel, C. S. (2019). iPSC- and mesenchymoangioblast-derived mesenchymal stem cells provide greater protection against experimental chronic allergic airways disease compared with a clinically used corticosteroid. The FASEB Journal, 33, 6402–6411.
Malaquias, M. A. S., et al. (2018). Effects of mesenchymal stromal cells play a role the oxidant/antioxidant balance in a murine model of asthma. Allergol Immunopathol (Madr), 46(136–143).
Duong, K. M., Arikkatt, J., Ullah, M. A., Lynch, J. P., Zhang, V., Atkinson, K., Sly, P. D., & Phipps, S. (2015). Immunomodulation of airway epithelium cell activation by mesenchymal stromal cells ameliorates house dust mite-induced airway inflammation in mice. American Journal of Respiratory Cell and Molecular Biology, 53, 615–624.
Zeng, S. L., Wang, L. H., Li, P., Wang, W., & Yang, J. (2015). Mesenchymal stem cells abrogate experimental asthma by altering dendritic cell function. Molecular Medicine Reports, 12, 2511–2520.
Keyhanmanesh, R., Rahbarghazi, R., Aslani, M. R., Hassanpour, M., & Ahmadi, M. (2018). Systemic delivery of mesenchymal stem cells condition media in repeated doses acts as magic bullets in restoring IFN-γ/IL-4 balance in asthmatic rats. Life Sciences, 212, 30–36.
Mirershadi, F., Ahmadi, M., Rezabakhsh, A., Rajabi, H., Rahbarghazi, R., & Keyhanmanesh, R. (2020). Unraveling the therapeutic effects of mesenchymal stem cells in asthma. Stem Cell Research & Therapy, 11, 400.
De Castro, L. L., et al. (2017). Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Research & Therapy, 8, 1–12.
Du, Y. (2018). Mo et al. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Experimental Cell Research, 363, 114–120.
Fang, S. B., Zhang, H. Y., Wang, C., He, B. X., Liu, X. Q., Meng, X. C., Peng, Y. Q., Xu, Z. B., Fan, X. L., Wu, Z. J., Chen, D., Zheng, L., Zheng G., & Fu Q. L. (2020). Small extracellular vesicles derived from human mesenchymal stromal cells prevent group 2 innate lymphoid cell-dominant allergic airway inflammation through delivery of miR-146a-5p. Journal of Extracellular Vesicles, 9, 1. https://doi.org/10.1080/20013078.2020.1723260.
Ahmad, T., Mukherjee, S., Pattnaik, B., Kumar, M., Singh, S., Kumar, M., Rehman, R., Tiwari, B. K., Jha, K. A., Barhanpurkar, A. P., Wani, M. R., Roy, S. S., Mabalirajan, U., Ghosh, B., & Agrawal, A. (2014). Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. The EMBO Journal, 33, 994–1010.
Agustí, A., & Hogg, J. C. (2019). Update on the pathogenesis of chronic obstructive pulmonary disease. The New England Journal of Medicine, 381, 1248–1256.
Vogelmeier, C. F., Criner, G. J., Martinez, F. J., Anzueto, A., Barnes, P. J., Bourbeau, J., Celli, B. R., Chen, R., Decramer, M., Fabbri, L. M., Frith, P., Halpin, D. M. G., López Varela, M. V., Nishimura, M., Roche, N., Rodriguez-Roisin, R., Sin, D. D., Singh, D., Stockley, R., Vestbo, J., Wedzicha, J. A., & Agustí, A. (2017). Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. American Journal of Respiratory and Critical Care Medicine, 195, 557–582.
Strategy, G., Obstructive, C., & Disease, P. (2017). GOLD (global initiative for chronic Obstructive lung Disease) 2017. Pneumologie, 71, 9–14.
Shigemura, N., Okumura, M., Mizuno, S., Imanishi, Y., Nakamura, T., & Sawa, Y. (2006). Autologous transplantation of adipose tissue-derived stromal cells ameliorates pulmonary emphysema. American Journal of Transplantation, 6, 2592–2600.
Kim, Y. S., Kokturk, N., Kim, J. Y., Lee, S. W., Lim, J., Choi, S. J., Oh, W., & Oh, Y. M. (2016). Gene profiles in a smoke-induced COPD mouse lung model following treatment with mesenchymal stem cells. Molecules and Cells, 39, 728–733.
Kim, Y. S., Kim, J. Y., Huh, J. W., Lee, S. W., Choi, S. J., & Oh, Y. M. (2015). The therapeutic effects of optimal dose of mesenchymal stem cells in a murine model of an elastase induced-emphysema. Tuberc. Respir. Dis. (Seoul)., 78(239–245), 239–245.
Takeda, K. et al. (2018) Activation of p70S6 Kinase-1 in Mesenchymal Stem Cells Is Essential to Lung Tissue Repair. Stem Cells Translational Medicine 551–558 https://doi.org/10.1002/sctm.17-0200.
Weiss, D. J., Casaburi, R., Flannery, R., LeRoux-Williams, M., & Tashkin, D. P. (2013). A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest, 143, 1590–1598.
Harrell, C. R., Miloradovic, D., Sadikot, R., Fellabaum, C., Markovic, B. S., Miloradovic, D., Acovic, A., Djonov, V., Arsenijevic, N., & Volarevic, V. (2020). Molecular and cellular mechanisms responsible for beneficial effects of Mesenchymal stem cell-derived product ‘exo-d-MAPPS’ in attenuation of chronic airway inflammation. Analytical Cellular Pathology, 2020, 1–15.
Kim, Y. S., et al. (2017). Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Experimental & Molecular Medicine, 49.
Li, X., et al. (2018). Mesenchymal stem cells alleviate oxidative stress–induced mitochondrial dysfunction in the airways. J. Allergy Clin. Immunol., 141, 1634–1645.e5.
Raghu, G., Collard, H. R., Egan, J. J., Martinez, F. J., Behr, J., Brown, K. K., Colby, T. V., Cordier, J. F., Flaherty, K. R., Lasky, J. A., Lynch, D. A., Ryu, J. H., Swigris, J. J., Wells, A. U., Ancochea, J., Bouros, D., Carvalho, C., Costabel, U., Ebina, M., Hansell, D. M., Johkoh, T., Kim, D. S., King te Jr, Kondoh, Y., Myers, J., Müller, N. L., Nicholson, A. G., Richeldi, L., Selman, M., Dudden, R. F., Griss, B. S., Protzko, S. L., Schünemann, H. J., & ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. (2011). An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. American Journal of Respiratory and Critical Care Medicine, 183, 788–824.
Ortiz, L. A., et al. (2003). Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proceedings of the National Academy of Sciences of the United States of America, 100, 8407–8411.
Moodley, Y., Atienza, D., Manuelpillai, U., Samuel, C. S., Tchongue, J., Ilancheran, S., Boyd, R., & Trounson, A. (2009). Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. The American Journal of Pathology, 175, 303–313.
Reddy, M., Fonseca, L., Gowda, S., Chougule, B., Hari, A., & Totey, S. (2016). Human adipose-derived mesenchymal stem cells attenuate early stage of bleomycin induced pulmonary fibrosis: Comparison with pirfenidone. Int. J. Stem Cells, 9, 192–206.
Ono, M., Ohkouchi, S., Kanehira, M., Tode, N., Kobayashi, M., Ebina, M., Nukiwa, T., Irokawa, T., Ogawa, H., Akaike, T., Okada, Y., Kurosawa, H., Kikuchi, T., & Ichinose, M. (2015). Mesenchymal stem cells correct inappropriate epithelial-mesenchyme relation in pulmonary fibrosis using stanniocalcin-1. Molecular Therapy, 23, 549–560.
Akram, K. M., Samad, S., Spiteri, M. A., & Forsyth, N. R. (2013). Mesenchymal stem cells promote alveolar epithelial cell wound repair in vitro through distinct migratory and paracrine mechanisms. Respiratory Research, 14, 1.
Liu, M., et al. (2016). Immunomodulation by mesenchymal stem cells in treating human autoimmune disease-associated lung fibrosis. Stem Cell Research & Therapy, 7, 1–15.
Li, F., Han, F., Li, H., Zhang, J., Qiao, X., Shi, J., Yang, L., Dong, J., Luo, M., Wei, J., & Liu, X. (2017). Human placental mesenchymal stem cells of fetal origins-alleviated inflammation and fibrosis by attenuating MyD88 signaling in bleomycin-induced pulmonary fibrosis mice. Molecular Immunology, 90, 11–21.
Gad, E. S., Salama, A. A. A., el-Shafie, M. F., Arafa, H. M. M., Abdelsalam, R. M., & Khattab, M. (2020). The anti-fibrotic and anti-inflammatory potential of bone marrow–derived Mesenchymal stem cells and Nintedanib in Bleomycin-induced lung fibrosis in rats. Inflammation, 43, 123–134.
Mansouri, N., et al. (2019). Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight, 4, 1–17.
Wan, X. et al. (2020) Mesenchymal stem cell-derived extracellular vesicles suppress the fibroblast proliferation by downregulating FZD6 expression in fibroblasts via micrRNA-29b-3p in idiopathic pulmonary fibrosis. Journal of Cellular Physiology. https://doi.org/10.1002/jcp.29706.
Thenappan, T., Ormiston, M. L., Ryan, J. J., & Archer, S. L. (2018). Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ (Online), j5492. https://doi.org/10.1136/bmj.j5492.
Rabinovitch, M., Guignabert, C., Humbert, M., & Nicolls, M. R. (2014). Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circulation Research, 115, 165–175. https://doi.org/10.1161/CIRCRESAHA.113.301141.
Baber, S. R., et al. (2007). Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am. J. Physiol. - Hear. Circ. Physiol., 292, 1120–1128.
Schmuck, E. G., Hacker, T. A., Schreier, D. A., Chesler, N. C., & Wang, Z. (2019). Beneficial effects of mesenchymal stem cell delivery via a novel cardiac bioscaffold on right ventricles of pulmonary arterial hypertensive rats. Am. J. Physiol. - Hear. Circ. Physiol., 316, H1005–H1013.
Luan, Y., Zhang, X., Qi, T. G., Cheng, G. H., Sun, C., & Kong, F. (2014). Long-term research of stem cells in monocrotaline-induced pulmonary arterial hypertension. Clinical and Experimental Medicine, 14, 439–446.
Badr Eslam, R., Croce, K., Mangione, F. M., Musmann, R., Leopold, J. A., Mitchell, R. N., & Waxman, A. B. (2017). Persistence and proliferation of human mesenchymal stromal cells in the right ventricular myocardium after intracoronary injection in a large animal model of pulmonary hypertension. Cytotherapy, 19, 668–679.
Tan, R., Li, J., Peng, X., Zhu, L., Cai, L., Wang, T., Su, Y., Irani, K., & Hu, Q. (2013). GAPDH is critical for superior efficacy of female bone marrow-derived mesenchymal stem cells on pulmonary hypertension. Cardiovascular Research, 100, 19–27.
Alencar, A. K. N., et al. (2018). Human Mesenchymal stem cell therapy reverses Su5416/hypoxia-induced pulmonary arterial hypertension in mice. Frontiers in Pharmacology, 9, 1–16.
Chou, H. C., Lin, W., & Chen, C. M. (2016). Human mesenchymal stem cells attenuate pulmonary hypertension induced by prenatal lipopolysaccharide treatment in rats. Clinical and Experimental Pharmacology & Physiology, 43, 906–914.
Cheng, G., Wang, X., Li, Y., & He, L. (2017). Let-7a-transfected mesenchymal stem cells ameliorate monocrotaline-induced pulmonary hypertension by suppressing pulmonary artery smooth muscle cell growth through STAT3-BMPR2 signaling. Stem Cell Research & Therapy, 8, 1–11.
De Mendonça, L., et al. (2017). Mesenchymal stromal cell therapy reduces lung inflammation and vascular remodeling and improves hemodynamics in experimental pulmonary arterial hypertension. Stem Cell Research & Therapy, 8, 1–15.
Huang, J., Lu, W., Ouyang, H., Chen, Y., Zhang, C., Luo, X., Li, M., Shu, J., Zheng, Q., Chen, H., Chen, J., Tang, H., Sun, D., Yuan, J. X., Yang, K., & Wang, J. (2020). Transplantation of mesenchymal stem cells attenuates pulmonary hypertension by normalizing the endothelial-to-mesenchymal transition. American Journal of Respiratory Cell and Molecular Biology, 62, 49–60.
Wang, P., Zhang, C., Li, J., Luo, L., Zhang, S., Dong, F., Tang, Z., & Ni, S. (2019). Adipose-derived mesenchymal stromal cells improve hemodynamic function in pulmonary arterial hypertension: Identification of microRNAs implicated in modulating endothelial function. Cytotherapy, 21, 416–427.
Tzouvelekis, A., et al. (2013). A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. Journal of Translational Medicine, 11, 1.
Chambers, D. C., Enever, D., Ilic, N., Sparks, L., Whitelaw, K., Ayres, J., Yerkovich, S. T., Khalil, D., Atkinson, K. M., & Hopkins, P. M. A. (2014). A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology, 19, 1013–1018.
Averyanov, A., Koroleva, I., Konoplyannikov, M., Revkova, V., Lesnyak, V., Kalsin, V., Danilevskaya, O., Nikitin, A., Sotnikova, A., Kotova, S., & Baklaushev, V. (2020). First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Translational Medicine, 9, 6–16.
Chen, J. Y., An, R., Liu, Z. J., Wang, J. J., Chen, S. Z., Hong, M. M., Liu, J. H., Xiao, M. Y., & Chen, Y. F. (2014). Therapeutic effects of mesenchymal stem cell-derived microvesicles on pulmonary arterial hypertension in rats. Acta Pharmacologica Sinica, 35, 1121–1128.
Klinger, J. R., Pereira, M., del Tatto, M., Brodsky, A. S., Wu, K. Q., Dooner, M. S., Borgovan, T., Wen, S., Goldberg, L. R., Aliotta, J. M., Ventetuolo, C. E., Quesenberry, P. J., & Liang, O. D. (2020). Mesenchymal stem cell extracellular vesicles reverse Sugen/hypoxia pulmonary hypertension in rats. American Journal of Respiratory Cell and Molecular Biology, 62, 577–587.
Zhang, S., et al. (2020). Mesenchymal stromal cell-derived exosomes improve pulmonary hypertension through inhibition of pulmonary vascular remodeling. Respiratory Research, 21, 1–12.
Lee, C., Mitsialis, S. A., Aslam, M., Vitali, S. H., Vergadi, E., Konstantinou, G., Sdrimas, K., Fernandez-Gonzalez, A., & Kourembanas, S. (2012). Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation, 126, 2601–2611.
Zhang, C., et al. (2019). Function of adipose-derived Mesenchymal stem cells in Monocrotaline-induced pulmonary arterial hypertension through miR-191 via regulation of BMPR2. BioMed Research International, 2019.
Liu, Z., Liu, J., Xiao, M., Wang, J., Yao, F., Zeng, W., Yu, L., Guan, Y., Wei, W., Peng, Z., Zhu, K., Wang, J., Yang, Z., Zhong, J., & Chen, J. (2018). Mesenchymal stem cell–derived microvesicles alleviate pulmonary arterial hypertension by regulating renin-angiotensin system. Journal of the American Society of Hypertension, 12, 470–478.
Hogan, S. E., Rodriguez Salazar, M. P., Cheadle, J., Glenn, R., Medrano, C., Petersen, T. H., & Ilagan, R. M. (2019). Mesenchymal stromal cell-derived exosomes improve mitochondrial health in pulmonary arterial hypertension. Am. J. Physiol. - Lung Cell. Mol. Physiol., 316, L723–L737.
The Lancet Respiratory Medicine. (2019). The world is failing on silicosis. The Lancet Respiratory Medicine, 7, 283. https://doi.org/10.1016/S2213-2600(19)30078-5.
Li, X., An, G., Wang, Y., Liang, D., Zhu, Z., Lian, X., Niu, P., Guo, C., & Tian, L. (2017). Anti-fibrotic effects of bone morphogenetic protein-7-modified bone marrow mesenchymal stem cells on silica-induced pulmonary fibrosis. Experimental and Molecular Pathology, 102, 70–77. https://doi.org/10.1016/j.yexmp.2016.12.010.
Jiang, R., Liao, Y., Yang, F., Cheng, Y., Dai, X., & Chao, J. (2019). SPIO nanoparticle-labeled bone marrow mesenchymal stem cells inhibit pulmonary EndoMT induced by SiO2. Experimental Cell Research, 383, 111492. https://doi.org/10.1016/j.yexcr.2019.07.005.
Chen, S., Cui, G., Peng, C., Lavin, M. F., Sun, X., Zhang, E., Yang, Y., Guan, Y., du, Z., & Shao, H. (2018). Transplantation of adipose-derived mesenchymal stem cells attenuates pulmonary fibrosis of silicosis via anti-inflammatory and anti-apoptosis effects in rats. Stem Cell Research & Therapy, 9, 110. https://doi.org/10.1186/s13287-018-0846-9.
Zhang, E., et al. (2018). Bone marrow mesenchymal stromal cells attenuate silica-induced pulmonary fibrosis potentially by attenuating Wnt/β-catenin signaling in rats. Stem Cell Research & Therapy, 9, 1–14.
Li, X., An, G., Wang, Y., Liang, D., Zhu, Z., & Tian, L. (2018). Targeted migration of bone marrow mesenchymal stem cells inhibits silica-induced pulmonary fibrosis in rats. Stem Cell Research & Therapy, 9, 335. https://doi.org/10.1186/s13287-018-1083-y.
Liu, W. W., Wang, H. X., Yu, W., Bi, X. Y., Chen, J. Y., Chen, L. Z., Ding, L., Han, D. M., Guo, Z. K., & Lei, Y. X. (2015). Treatment of silicosis with hepatocyte growth factor-modified autologous bone marrow stromal cells: A non-randomized study with follow-up. Genetics and Molecular Research, 14, 10672–10681. https://doi.org/10.4238/2015.September.9.7.
Bandeira, E., Oliveira, H., Silva, J. D., Menna-Barreto, R. F. S., Takyia, C. M., Suk, J. S., Witwer, K. W., Paulaitis, M. E., Hanes, J., Rocco, P. R. M., & Morales, M. M. (2018). Therapeutic effects of adipose-tissue-derived mesenchymal stromal cells and their extracellular vesicles in experimental silicosis. Respiratory Research, 19, 104. https://doi.org/10.1186/s12931-018-0802-3.
Phinney, D. G., di Giuseppe, M., Njah, J., Sala, E., Shiva, S., St Croix, C. M., Stolz, D. B., Watkins, S. C., di, Y. P., Leikauf, G. D., Kolls, J., Riches, D. W. H., Deiuliis, G., Kaminski, N., Boregowda, S. V., McKenna, D. H., & Ortiz, L. A. (2015). Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nature Communications, 6, 8472.
Choi, M., Ban, T. & Rhim, T. (2014) Therapeutic use of stem cell transplantation for cell replacement or cytoprotective effect of microvesicle released from mesenchymal stem cell. Molecular Cell. https://doi.org/10.14348/molcells.2014.2317.
Witwer, K. W., van Balkom, B. W. M., Bruno, S., Choo, A., Dominici, M., Gimona, M., Hill, A. F., de Kleijn, D., Koh, M., Lai, R. C., Mitsialis, S. A., Ortiz, L. A., Rohde, E., Asada, T., Toh, W. S., Weiss, D. J., Zheng, L., Giebel, B., & Lim, S. K. (2019). Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles, 8.
Calfee, C. S., Delucchi, K., Parsons, P. E., Thompson, B. T., Ware, L. B., Matthay, M. A., & NHLBI ARDS Network. (2014). Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. The Lancet Respiratory Medicine, 2, 611–620.
Camussi, G. & Quesenberry, J, P. (2013) Perspectives on the Potential Therapeutic Uses of Vesicles. Exosomes and Microvesicles. https://doi.org/10.5772/57393.
Chen, T. S., et al. (2011). Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. Journal of Translational Medicine, 9, 47. https://doi.org/10.1186/1479-5876-9-47.
Pachler, K., Lener, T., Streif, D., Dunai, Z. A., Desgeorges, A., Feichtner, M., Öller, M., Schallmoser, K., Rohde, E., & Gimona, M. (2017). A good manufacturing practice–grade standard protocol for exclusively human mesenchymal stromal cell–derived extracellular vesicles. Cytotherapy, 19, 458–472.
Mendt, M., et al. (2018). Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI insight, 3, 1–22.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
No financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Additional information
This article belongs to the Topical Collection: Special Issue on Exosomes and Microvesicles: from Stem Cell Biology to Translation in Human Diseases
Guest Editor: Giovanni Camussi
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, H., Su, Y. & Deng, F. Effects of Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Lung Diseases: Current Status and Future Perspectives. Stem Cell Rev and Rep 17, 440–458 (2021). https://doi.org/10.1007/s12015-020-10085-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-10085-8