Abstract
Stems cells of the colon crypt are the origin of colon mature cells. Colorectal cancer cells are also suggested to originate from crypt stem cells undergoing a series of epigenetic and genetic alterations. Aberrant methylation plays important roles in early carcinogenesis and lead to altered gene expression and regulation, resulting in accumulation of damages to cell function and ultimately, malignant transformation. Aberrances in hypermethylation and hypomethylation act in different mechanism through the regulation of various genes during CSC carcinogenesis, and both of them play crucial roles in stem cell differentiation towards cancer cells. A large majority of epigenetic and genetic abnormalities that work coordinately in colorectal carcinogenesis are related to cell growth and division, indicating that the intrinsic abnormalities of CRC lie in dysregulation of basic cellular processes. Detection of abnormal methylation can be used in cancer screening and early detection, while reversal of aberrant methylation using drugs may have potential in cancer therapy. This review will provide an overview on the roles of aberrant methylation and a summary of genes that are affected during CRC carcinogenesis.


Similar content being viewed by others
References
Jaenisch, R., & Bird, A. (2003). Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics, 33, 245–254.
Jones, P. A., & Baylin, S. B. (2007). The epigenomics of cancer. Cell, 128, 683–692.
Roostaee, A., Benoit, Y. D., Boudjadi, S., & Beaulieu, J. F. (2016). Epigenetics in intestinal epithelial cell renewal. Journal of Cellular Physiology, 2016.
Houghton, J., Morozov, A., Smirnova, I., & Wang, T. C. (2006). Stem cells and cancer. Seminars in Cancer Biology, 2007(17), 191–203.
Lapidot, T., Sirard, C., Vormoor, J., et al. (1994). A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature, 367, 645–648.
Bonnet, D., & Dick, J. E. (1997). Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine, 3, 730–737.
Lobo, N. A., Shimono, Y., Qian, D., & Clarke, M. F. (2007). The biology of cancer stem cells. Annual Review of Cell and Developmental Biology, 23, 675–699.
Potten, C. S., Gandara, R., Mahida, Y. R., Loeffler, M., & Wright, N. A. (2009). The stem cells of small intestinal crypts: where are they? Cell Proliferation, 42, 731–750.
Medema, J. P., & Vermeulen, L. (2011). Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature, 474, 318–326.
Markowitz, S. D., & Bertagnolli, M. M. (2009). Molecular origins of cancer: molecular basis of colorectal cancer. The New England Journal of Medicine, 361, 2449–2460.
Baylin, S. B., & Ohm, J. E. (2006). Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nature Reviews. Cancer, 6, 107–116.
Song, L., Li, Y., He, B., & Gong, Y. (2015). Development of small molecules targeting the Wnt signaling pathway in cancer stem cells for the treatment of colorectal cancer. Clinical Colorectal Cancer, 14, 133–145.
Jones, P. L., Veenstra, G. J., Wade, P. A., et al. (1998). Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nature Genetics, 19, 187–191.
Nan, X., Ng, H. H., Johnson, C. A., et al. (1998). Transcriptionalrepression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature, 393, 386–389.
Irizarry, R. A., Ladd-Acosta, C., Wen, B., et al. (2009). The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nature Genetics, 41, 178–186.
Elliott, E. N., & Kaestner, K. H. (2015). Epigenetic regulation of the intestinal epithelium. Cellular and Molecular Life Sciences, 72, 4139–4156.
Fearon, E. R. (2011). Molecular genetics of colorectal cancer. Annual Review of Pathology, 6, 479–507.
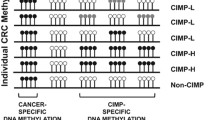
Toyota, M., Ahuja, N., Ohe-Toyota, M., Herman, J. G., Baylin, S. B., & Issa, J. P. (1999). CpG island methylator phenotype in colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America, 96, 8681–8686.
Weisenberger, D. J., Siegmund, K. D., Campan, M., et al. (2006). CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nature Genetics, 38, 787–793.
Network, C. G. A. (2012). Comprehensive molecular characterization of human colon and rectal cancer. Nature, 487, 330–337.
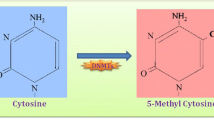
Rhee, I., Bachman, K. E., Park, B. H., et al. (2002). DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature, 416, 552–556.
Robert, M. F., Morin, S., Beaulieu, N., et al. (2003). DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nature Genetics, 33, 61–65.
Hammoud, S. S., Cairns, B. R., & Jones, D. A. (2013). Epigenetic regulation of colon cancer and intestinal stem cells. Current Opinion in Cell Biology, 25, 177–183.
Feinberg, A. P., & Vogelstein, B. (1983). Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature, 301, 89–92.
Gama-Sosa, M. A., Slagel, V. A., Trewyn, R. W., et al. (1983). The 5-methylcytosine content of DNA from human tumors. Nucleic Acids Research, 11, 6883–6894.
Goelz, S. E., Vogelstein, B., Hamilton, S. R., & Feinberg, A. P. (1985). Hypomethylation of DNA from benign and malignant human colon neoplasms. Science, 228, 187–190.
Feinberg, A. P., Gehrke, C. W., Kuo, K. C., & Ehrlich, M. (1988). Reduced genomic 5-methylcytosine content in human colonic neoplasia. Cancer Research, 48, 1159–1161.
Chen, R. Z., Pettersson, U., Beard, C., Jackson-Grusby, L., & Jaenisch, R. (1998). DNA hypomethylation leads to elevated mutation rates. Nature, 395, 89–93.
Nakamura, N., & Takenaga, K. (1998). Hypomethylation of the metastasis-associated S100 A4 gene correlates with gene activation in human colon adenocarcinoma cell lines. Clinical & Experimental Metastasis, 16, 471–479.
Lengauer, C., Kinzler, K. W., & Vogelstein, B. (1997). DNA methylation and genetic instability in colorectal cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 94, 2545–2550.
Eads, C. A., Danenberg, K. D., Kawakami, K., Saltz, L. B., Danenberg, P. V., & Laird, P. W. (1999). CpG island hypermethylation in human colorectal tumors is not associated with DNA methyltransferase overexpression. Cancer Research, 59, 2302–2306.
Cormier, R. T., & Dove, W. F. (2000). Dnmt1N/+ reduces the net growth rate and multiplicity of intestinal adenomas in C57BL/6-multiple intestinal neoplasia (Min)/+ mice independently of p53 but demonstrates strong synergy with the modifier of Min 1(AKR) resistance allele. Cancer Research, 60, 3965–3970.
Eads, C. A., Nickel, A. E., & Laird, P. W. (2002). Complete genetic suppression of polyp formation and reduction of CpG-island hypermethylation in Apc(Min/+) Dnmt1-hypomorphic mice. Cancer Research, 62, 1296–1299.
Loriot, A., Parvizi, G. K., Reister, S., & De Smet, C. (2012). Silencing of cancer-germline genes in human preimplantation embryos: evidence for active de novo DNA methylation in stem cells. Biochemical and Biophysical Research Communications, 417(1), 187–191.
Trowbridge, J. J., Sinha, A. U., Zhu, N., Li, M., Armstrong, S. A., & Orkin, S. H. (2012). Haploinsufficiency of Dnmt1 impairs leukemia stem cell function through derepression of bivalent chromatin domains. Genes & Development, 26, 344–349.
Trowbridge, J. J., & Orkin, S. H. (2011). Dnmt3a silences hematopoietic stem cell self-renewal. Nature Genetics, 44, 13–14.
Zhang, R. P., Shao, J. Z., & Xiang, L. X. (2011). GADD45A protein plays an essential role in active DNA demethylation during terminal osteogenic differentiation of adipose-derived mesenchymal stem cells. The Journal of Biological Chemistry, 286, 41083–41094.
Zhuang, J., Jones, A., Lee, S. H., et al. (2012). The dynamics and prognostic potential of DNA methylation changes at stem cell gene loci in women's cancer. PLoS Genetics, 8, e1002517.
Nazor, K. L., Altun, G., Lynch, C., et al. (2012). Recurrent variations in DNA methylation in human pluripotent stem cells and their differentiated derivatives. Cell Stem Cell, 10, 620–634.
The, M. T., Gemenetzidis, E., Patel, D., et al. (2012). FOXM1 induces a global methylation signature that mimics the cancer epigenome in head and neck squamous cell carcinoma. PloS One, 7, e34329.
Tsujii, M. (2013). Cyclooxygenase, cancer stem cells and DNA methylation play important roles in colorectal carcinogenesis. Digestion, 87, 12–16.
Migliore, L., Migheli, F., Spisni, R., & Coppedè, F. (2011). Genetics, cytogenetics, and epigenetics of colorectal cancer. Journal of Biomedicine & Biotechnology, 2011, 792362.
Migheli, F., & Migliore, L. (2011). Epigenetics of colorectal cancer. Clinical Genetics, 81, 312–318.
Ku, J. L., Kang, S. B., Shin, Y. K., et al. (2004). Promoter hypermethylation downregulates RUNX3 gene expression in colorectal cancer cell lines. Oncogene, 23, 6736–6742.
Goel, A., Arnold, C. N., Tassone, P., et al. (2004). Epigenetic inactivation of RUNX3 in microsatellite unstable sporadic colon cancers. International Journal of Cancer, 112, 754–759.
Tan, S. H., Ida, H., Lau, Q. C., et al. (2007). Detection of promoter hypermethylation in serum samples of cancer patients by methylation-specific polymerase chain reaction for tumour suppressor genes including RUNX3. Oncology Reports, 18, 1225–1230.
Kawakami, K., Ruszkiewicz, A., Bennett, G., Moore, J., Watanabe, G., & Iacopetta, B. (2003). The folate pool in colorectal cancers is associated with DNA hypermethylation and with a polymorphism in methylenetetrahydrofolate reductase. Clinical Cancer Research, 9, 5860–5865.
Lee, S., Hwang, K. S., Lee, H. J., Kim, J. S., & Kang, G. H. (2004). Aberrant CpG island hypermethylation of multiple genes in colorectal neoplasia. Laboratory Investigation, 84, 884–893.
Gryfe, R., Swallow, C., Bapat, B., Redston, M., Gallinger, S., & Couture, J. (1997). Molecular biology of colorectal cancer. Current Problems in Cancer, 21, 233–300.
Liang, J. T., Chang, K. J., Chen, J. C., et al. (1999). Hypermethylation of the p16 gene in sporadic T3N0M0 stage colorectal cancers: association with DNA replication error and shorter survival. Oncology, 57, 149–156.
Strathdee, G., Appleton, K., Illand, M., et al. (2001). Primary ovarian carcinomas display multiple methylator phenotypes involving known tumor suppressor genes. The American Journal of Pathology, 158, 1121–1127.
Xu, X. L., Yu, J., & Zhang, H. Y. (2004). Methylation profile of the promoter CpG islands of 31 genes that may contribute to colorectal carcinogenesis. World Journal of Gastroenterology, 10, 3441–3454.
Mossman, D., & Scott, R. J. (2011). Long term transcriptional reactivation of epigenetically silenced genes in colorectal cancer cells requires DNA hypomethylation and histone acetylation. PloS One, 6, e23127.
van Engeland, M., Roemen, G. M., Brink, M., et al. (2002). K-ras mutations and RASSF1A promoter methylation in colorectal cancer. Oncogene, 21, 3792–3795.
Wagner, K. J., Cooper, W. N., Grundy, R. G., et al. (2002). Frequent RASSF1A tumour suppressor gene promoter methylation in Wilms' tumour and colorectal cancer. Oncogene, 21, 7277–7282.
Esteller, M., Tortola, S., Toyota, M., et al. (2000). Hypermethylation-associated inactivation of p14(ARF) is independent of p16(INK4a) methylation and p53 mutational status. Cancer Research, 60, 129–133.
Corn, P. G., Summers, M. K., Fogt, F., et al. (2003). Frequent hypermethylation of the 5' CpG island of the mitotic stress checkpoint gene Chfr in colorectal and non-small cell lung cancer. Carcinogenesis, 24, 47–51.
Brandes, J. C., van Engeland, M., Wouters, K. A., Weijenberg, M. P., & Herman, J. G. (2005). CHFR promoter hypermethylation in colon cancer correlates with the microsatellite instability phenotype. Carcinogenesis, 26, 1152–1156.
Zhang, Y., Ye, X., Geng, J., & Chen, L. (2010). Epigenetic inactivation of deleted in lung and esophageal cancer 1 gene by promoter methylation in gastric and colorectal adenocarcinoma. Hepato-Gastroenterology, 57, 1614–1619.
Guo, Y., Shu, L., Zhang, C., Su, Z. Y., & Kong, A. N. (2015). Curcumin inhibits anchorage-independent growth of HT29 human colon cancer cells by targeting epigenetic restoration of the tumor suppressor gene DLEC1. Biochemical Pharmacology, 94, 69–78.
Hiranuma, C., Kawakami, K., Oyama, K., Ota, N., Omura, K., & Watanabe, G. (2004). Hypermethylation of the MYOD1 gene is a novel prognostic factor in patients with colorectal cancer. International Journal of Molecular Medicine, 13, 413–417.
Arasaradnam, R. P., Quraishi, M. N., Commane, D., Mathers, J. C., & Bradburn, M. (2012). MYOD-1 in normal colonic mucosa--role as a putative biomarker? BMC Research Notes, 5, 240.
Fosbrink, M., Cudrici, C., Niculescu, F., et al. (2005). Overexpression of RGC-32 in colon cancer and other tumors. Experimental and Molecular Pathology, 78, 116–122.
Vlaicu, S. I., Tegla, C. A., Cudrici, C. D., et al. (2010). Epigenetic modifications induced by RGC-32 in colon cancer. Experimental and Molecular Pathology, 88, 67–76.
Kim, Y. I., Pogribny, I. P., Salomon, R. N., et al. (1996). Exon-specific DNA hypomethylation of the p53 gene of rat colon induced by dimethylhydrazine. Modulation by dietary folate. The American Journal of Pathology, 149, 1129–1137.
Wasson, G. R., McGlynn, A. P., McNulty, H., et al. (2006). Global DNA and p53 region-specific hypomethylation in human colonic cells is induced by folate depletion and reversed by folate supplementation. The Journal of Nutrition, 136, 2748–2753.
Luo, L., Chen, W. D., & Pretlow, T. P. (2005). CpG island methylation in aberrant crypt foci and cancers from the same patients. International Journal of Cancer, 115, 747–751.
Choi, J. S., Kim, K. H., & Jeon, Y. K. (2009). Promoter hypermethylation of the ADAM23 gene in colorectal cancer cell lines and cancer tissues. International Journal of Cancer, 124, 1258–1262.
Sandhu, S., Wu, X., & Nabi, Z. (2012). Loss of HLTF function promotes intestinal carcinogenesis. Molecular Cancer, 11, 18.
Smirnov, D. A., Foulk, B. W., Doyle, G. V., Connelly, M. C., Terstappen, L. W., & O'Hara, S. M. (2006). Global gene expression profiling of circulating endothelial cells in patients with metastatic carcinomas. Cancer Research, 66, 2918–2922.
Ogino, S., Kawasaki, T., Nosho, K., et al. (2008). LINE-1 hypomethylation is inversely associated with microsatellite instability and CpG island methylator phenotype in colorectal cancer. International Journal of Cancer, 122, 2767–2773.
Kawakami, K., Matsunoki, A., Kaneko, M., Saito, K., Watanabe, G., & Minamoto, T. (2011). Long interspersed nuclear element-1 hypomethylation is a potential biomarker for the prediction of response to oral fluoropyrimidines in microsatellite stable and CpG island methylator phenotype-negative colorectal cancer. Cancer Science, 102, 166–174.
Belkhiri, A., & El-Rifai, W. (2014). 5-methylcytosine hydroxylation-mediated LINE-1 hypomethylation: a novel mechanism of proto-oncogenes activation in colorectal cancer? Gut, 63, 538–539.
Tang, J. T., Wang, Z. H., & Fang, J. Y. (2015). Assessing the potential value of long interspersed element-1 hypomethylation in colorectal cancer: evidence from retrospective studies. Onco Targets and Theraphy, 8, 3265–3276.
Bernet, A., Mazelin, L., Coissieux, M. M., et al. (2007). Inactivation of the UNC5C netrin-1 receptor is associated with tumor progression in colorectal malignancies. Gastroenterology, 133, 1840–1848.
Shin, S. K., Nagasaka, T., Jung, B. H., et al. (2007). Epigenetic and genetic alterations in netrin-1 receptors UNC5C and DCC in human colon cancer. Gastroenterology, 133, 1849–1857.
Hibi, K., Mizukami, H., Shirahata, A., Goto, T., Sakata, M., & Sanada, Y. (2009). Aberrant methylation of the netrin-1 receptor genes UNC5C and DCC detected in advanced colorectal cancer. World Journal of Surgery, 33, 1053–1057.
Mokarram, P., Kumar, K., Brim, H., et al. (2009). Distinct high-profile methylated genes in colorectal cancer. PloS One, 4, e7012.
Gerecke, C., Scholtka, B., Löwenstein, Y., et al. (2015). Hypermethylation of ITGA4, TFPI2 and VIMENTIN promoters is increased in inflamed colon tissue: putative risk markers for colitis-associated cancer. Journal of Cancer Research and Clinical Oncology, 141, 2097–2107.
Rasmussen, S. L., Krarup, H. B., Sunesen, K. G., Pedersen, I. S., Madsen, P. H., & Thorlacius-Ussing, O. (2016). Hypermethylated DNA as a biomarker for colorectal cancer: a systematic review. Colorectal Disease, 18, 549–561.
Yi, J. M., Dhir, M., Van Neste, L., et al. (2011). Genomic and epigenomic integration identifies a prognostic signature in colon cancer. Clinical Cancer Research, 17, 1535–1545.
Grützmann, R., Molnar, B., Pilarsky, C., et al. (2008). Sensitive detection of colorectal cancer in peripheral blood by septin 9 DNA methylation assay. PloS One, 3, e3759.
Wasserkort, R., Kalmar, A., Valcz, G., et al. (2013). Aberrant septin 9 DNA methylation in colorectal cancer is restricted to a single CpG island. BMC Cancer, 13, 398.
Menigatti, M., Pedroni, M., Verrone, A. M., et al. (2007). O6-methylguanine-DNA methyltransferase promoter hypermethylation in colorectal carcinogenesis. Oncology Reports, 17, 1421–1427.
Esteller, M., Hamilton, S. R., Burger, P. C., Baylin, S. B., & Herman, J. G. (1999). Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Research, 59, 793–797.
Esteller, M., Risques, R. A., Toyota, M., et al. (2001). Promoter hypermethylation of the DNA repair gene O(6)-methylguanine-DNA methyltransferase is associated with the presence of G:C to a:T transition mutations in p53 in human colorectal tumorigenesis. Cancer Research, 61, 4689–4692.
Cunningham, J. M., Christensen, E. R., Tester, D. J., et al. (1998). Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Research, 58, 3455–3460.
Wheeler, J. M., Beck, N. E., Kim, H. C., Tomlinson, I. P., Mortensen, N. J., & Bodmer, W. F. (1999). Mechanisms of inactivation of mismatch repair genes in human colorectal cancer cell lines: the predominant role of hMLH1. Proceedings of the National Academy of Sciences of the United States of America, 96, 10296–10301.
Wheeler, J. M., Loukola, A., Aaltonen, L. A., Mortensen, N. J., & Bodmer, W. F. (2000). The role of hypermethylation of the hMLH1 promoter region in HNPCC versus MSI+ sporadic colorectal cancers. Journal of Medical Genetics, 37, 588–592.
Balbinotti, R. A., Ribeiro, U., Sakai, P., et al. (2007). hMLH1, hMSH2 and cyclooxygenase-2 (cox-2) in sporadic colorectal polyps. Anticancer Research, 27, 4465–4471.
Oue, N., Motoshita, J., Yokozaki, H., et al. (2002). Distinct promoter hypermethylation of p16INK4a, CDH1, and RAR-beta in intestinal, diffuse-adherent, and diffuse-scattered type gastric carcinomas. The Journal of Pathology, 198, 55–59.
Ando, T., Sugai, T., Habano, W., Jiao, Y. F., & Suzuki, K. (2005). Analysis of SMAD4/DPC4 gene alterations in multiploid colorectal carcinomas. Journal of Gastroenterology, 40, 708–715.
Sameer, A. S., & Siddiqi, M. A. (2011). SMAD4 promoter hypermethylation in Kashmiri colorectal cancer cases. Saudi Journal of Gastroenterology, 17, 297.
Bai, A. H., Tong, J. H., To, K.F., et al. (2004). Promoter hypermethylation of tumor-related genes in the progression of colorectal neoplasia. International Journal of Cancer, 112, 846–853.
Abdel-Rahman, W. M., Nieminen, T. T., Shoman, S., Eissa, S., & Peltomaki, P. (2014). Loss of p15INK4b expression in colorectal cancer is linked to ethnic origin. Asian Pacific Journal of Cancer Prevention, 15, 2083–2087.
Hibi, K., Sakata, M., Sakuraba, K., et al. (2008). Aberrant methylation of the HACE1 gene is frequently detected in advanced colorectal cancer. Anticancer Research, 28, 1581–1584.
Sakata, M., Kitamura, Y. H., Sakuraba, K., et al. (2009). Methylation of HACE1 in gastric carcinoma. Anticancer Research, 29, 2231–2233.
Nagasaka, T., Sasamoto, H., Notohara, K., et al. (2004). Colorectal cancer with mutation in BRAF, KRAS, and wild-type with respect to both oncogenes showing different patterns of DNA methylation. Journal of Clinical Oncology, 22, 4584–4594.
Castells, A., Payá, A., Alenda, C., et al. (2006). Cyclooxygenase 2 expression in colorectal cancer with DNA mismatch repair deficiency. Clinical Cancer Research, 12, 1686–1692.
Wheeler, J. M., Kim, H. C., Efstathiou, J. A., Ilyas, M., Mortensen, N. J., & Bodmer, W. F. (2001). Hypermethylation of the promoter region of the E-cadherin gene (CDH1) in sporadic and ulcerative colitis associated colorectal cancer. Gut, 48, 367–371.
Walsh, M. D., Clendenning, M., Williamson, E., et al. (2013). Expression of MUC2, MUC5AC, MUC5B, and MUC6 mucins in colorectal cancers and their association with the CpG island methylator phenotype. Modern Pathology, 26, 1642–1656.
Renaud, F., Mariette, C., Vincent, A., et al. (2016). The serrated neoplasia pathway of colorectal tumors: identification of MUC5AC hypomethylation as an early marker of polyps with malignant potential. International Journal of Cancer, 138, 1472–1481.
Kawamura, Y. I., Toyota, M., Kawashima, R., et al. (2008). DNA hypermethylation contributes to incomplete synthesis of carbohydrate determinants in gastrointestinal cancer. Gastroenterology, 135, 142–151.e3.
Hiltunen, M. O., Alhonen, L., Koistinaho, J., et al. (1997). Hypermethylation of the APC (adenomatous polyposis coli) gene promoter region in human colorectal carcinoma. International Journal of Cancer, 70, 644–648.
Kim, J. C., Koo, K. H., Roh, S. A., et al. (2003). Genetic and epigenetic changes in the APC gene in sporadic colorectal carcinoma with synchronous adenoma. International Journal of Colorectal Disease, 18, 203–209.
Arnold, C. N., Goel, A., Niedzwiecki, D., et al. (2004). APC promoter hypermethylation contributes to the loss of APC expression in colorectal cancers with allelic loss on 5q. Cancer Biology & Therapy, 3, 960–964.
Hiltunen, M. O., Koistinaho, J., Alhonen, L., et al. (1997). Hypermethylation of the WT1 and calcitonin gene promoter regions at chromosome 11p in human colorectal cancer. British Journal of Cancer, 76, 1124–1130.
Zhang, H., Zhu, Y. Q., Wu, Y. Q., Zhang, P., & Qi, J. (2014). Detection of promoter hypermethylation of Wnt antagonist genes in fecal samples for diagnosis of early colorectal cancer. World Journal of Gastroenterology, 20, 6329–6335.
Ostman, A., & Augsten, M. (2009). Cancer-associated fibroblasts and tumor growth–bystanders turning into key players. Current Opinion in Genetics & Development, 19, 67–73.
Mishra, P. J., Mishra, P. J., Humeniuk, R., et al. (2008). Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Research, 68, 4331–4339.
Mader, E. K., Maeyama, Y., Lin, Y., et al. (2009). Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clinical Cancer Research, 15, 7246–7255.
Spaeth, E. L., Dembinski, J. L., Sasser, A. K., et al. (2009). Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PloS One, 4, e4992.
Zeki, S. S., Graham, T. A., & Wright, N. A. (2011). Stem cells and their implications for colorectal cancer. Nature Reviews. Gastroenterology & Hepatology, 8, 90–100.
Kong, D., Li, Y., Wang, Z., & Sarkar, F. H. (2011). Cancer stem cells and epithelial-to-mesenchymal transition (EMT)-phenotypic cells: are they cousins or twins? Cancers (Basel), 3, 716–729.
Thiery, J. P., Acloque, H., Huang, Y. J. R., & Nieto, M. A. (2009). Epithelial-mesenchymal transitions in development and disease. Cell, 139, 871–890.
Galván, J. A., Helbling, M., Koelzer, V. H., et al. (2015). TWIST1 and TWIST2 promoter methylation and protein expression in tumor stroma influence the epithelial-mesenchymal transition-like tumor budding phenotype in colorectal cancer. Oncotarget, 6, 874–885.
Tange, S., Oktyabri, D., Terashima, M., Ishimura, A., & Suzuki, T. (2014). JARID2 is involved in transforming growth factor-beta-induced epithelial-mesenchymal transition of lung and colon cancer cell lines. PloS One, 9, e115684.
Valcz, G., Patai, A. V., Kalmár, A., et al. (2014). Myofibroblast-derived SFRP1 as potential inhibitor of colorectal carcinoma field effect. PloS One, 9, e106143.
Weygant, N., Qu, D., May, R., et al. (2015). DCLK1 is a broadly dysregulated target against epithelial-mesenchymal transition, focal adhesion, and stemness in clear cell renal carcinoma. Oncotarget, 6, 2193–2205.
Kang, X. C., Chen, M. L., Yang, F., et al. (2016). Promoter methylation and expression of SOCS-1 affect clinical outcome and epithelial-mesenchymal transition in colorectal cancer. Biomedicine & Pharmacotherapy, 80, 23–29.
Song, L., & Li, Y. (2015). SEPT9: a specific circulating biomarker for colorectal cancer. Advances in Clinical Chemistry, 72, 171–204.
Li, Y., Song, L., Gong, Y., & He, B. (2014). Detection of colorectal cancer by DNA methylation biomarker SEPT9: past, present and future. Biomarkers in Medicine, 8, 755–769.
Greenspan, E. J., Jablonski, M. A., Rajan, T. V., Levine, J., Belinsky, G. S., & Rosenberg, D. W. (2006). Epigenetic alterations in RASSF1A in human aberrant crypt foci. Carcinogenesis, 27, 1316–1322.
Abouzeid, H. E., Kassem, A. M., Abdel Wahab, A. H., El-mezayen, H. A., Sharad, H., & Abdel Rahman, S. (2011). Promoter hypermethylation of RASSF1A, MGMT, and HIC-1 genes in benign and malignant colorectal tumors. Tumour Biology, 32, 845–852.
Cassinotti, E., Melson, J., Liggett, T., et al. (2012). DNA methylation patterns in blood of patients with colorectal cancer and adenomatous colorectal polyps. International Journal of Cancer, 131, 1153–1157.
Kriegl, L., Neumann, J., Vieth, M., et al. (2011). Up and downregulation of p16 (Ink4a) expression in BRAF-mutated polyps/adenomas indicates a senescence barrier in the serrated route to colon cancer. Modern Pathology, 24, 1015–1022.
Minoo, P., Baker, K., Goswami, R., et al. (2006). Extensive DNA methylation in normal colorectal mucosa in hyperplastic polyposis. Gut, 55, 1467–1474.
Sakamoto, J., Fujiya, M., Okamoto, K., et al. (2010). Immunoprecipitation of nucleosomal DNA is a novel procedure to improve the sensitivity of serum screening for the p16 hypermethylation associated with colon cancer. Cancer Epidemiology, 34, 194–199.
Zheng, Y., Zhang, Y. W., & Chen, L. B. (2010). Analysis of RUNX3 promoter hypermethylation in the serum DNA of gastric and colorectal adenocarcinoma patients. Journal of Medical Postgraduates, 3, 016.
Ye, X. B., Zhang, Y. W., & Chen, L. B. (2010). Methylation status of DLEC1 promoter in colorectal cancer patients and its clinical relevance. Academic Journal of Second Military Medical University Acad, 31, 842.
Church, T. R., Wandell, M., Lofton-Day, C., et al. (2014). Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut, 63, 317–325.
Potter, N. T., Hurban, P., White, M. N., et al. (2014). Validation of a real-time PCR-based qualitative assay for the detection of methylated SEPT9 DNA in human plasma. Clinical Chemistry, 60, 1183–1191.
Li, H., Chiappinelli, K. B., Guzzetta, A. A., et al. (2014). Immune regulation by low doses of the DNA methyltransferase inhibitor 5-azacitidine in common human epithelial cancers. Oncotarget, 5, 587–598.
His, L. C., Xi, X., Wu, Y., & Lippman, S. M. (2005). The methyltransferase inhibitor 5-aza-2-deoxycytidine induces apoptosis via induction of 15-lipoxygenase-1 in colorectal cancer cells. Molecular Cancer Therapeutics, 4, 1740–1746.
Acknowledgments
This work was supported by the Beijing Municipal Science and Technology Project (capital public health project) No. Z151100003915092 sponsored by the Beijing Municipal Science and Technology Commission.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interests
The authors claim no conflict of interest.
Rights and permissions
About this article
Cite this article
Song, L., Li, Y. The Role of Stem Cell DNA Methylation in Colorectal Carcinogenesis. Stem Cell Rev and Rep 12, 573–583 (2016). https://doi.org/10.1007/s12015-016-9672-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-016-9672-6