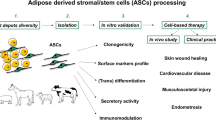
Abstract
Adipose-derived stromal/stem cells (ASC) are multipotent with abilities to differentiate into multiple lineages including connective tissue and neural cells. Despite unlimited opportunity and needs for human and veterinary regenerative medicine, applications of adipose-derived stromal/stem cells are at present very limited. Furthermore, the fundamental biological factors regulating stemness in ASC and their stable differentiation into other tissue cells are not fully understood. The objective of this review was to provide an update on the current knowledge of the nature and isolation, molecular and epigenetic determinants of the potency, and applications of adipose-derived stromal/stem cells, as well as challenges and future directions. The first quarter of the review focuses on the nature of ASC, namely their definition, origin, isolation and sorting methods and multilineage differentiation potential, often with a comparison to mesenchymal stem cells of bone marrow. Due to the indisputable role of epigenetic regulation on cell identities, epigenetic modifications (DNA methylation, chromatin remodeling and microRNAs) are described broadly in stem cells but with a focus on ASC. The final sections provide insights into the current and potential applications of ASC in human and veterinary regenerative medicine.


Similar content being viewed by others
References
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676.
da Silva Meirelles, L., Chagastelles, P. C., & Nardi, N. B. (2006). Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of Cell Science, 119(Pt 11), 2204–2213.
Dominici, M., Le Blanc, K., Mueller, I., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy, 8(4), 315–317.
Mizuno, H. (2009). Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. Journal of Nippon Medical School Nippon Ika Daigaku Zasshi, 76(2), 56–66.
Fraser, J. K., Schreiber, R., Strem, B., et al. (2006). Plasticity of human adipose stem cells toward endothelial cells and cardiomyocytes. Nature Clinical Practice. Cardiovascular Medicine, 3(Suppl 1), S33–S37.
Gimble, J., & Guilak, F. (2003). Adipose-derived adult stem cells: ısolation, characterization, and differentiation potential. Cytotherapy, 5(5), 362–369.
Gimble, J. M., Katz, A. J., & Bunnell, B. A. (2007). Adipose-derived stem cells for regenerative medicine. Circulation Research, 100(9), 1249–1260.
Gir, P., Oni, G., Brown, S. A., Mojallal, A., & Rohrich, R. J. (2012). Human adipose stem cells: current clinical applications. Plastic and Reconstructive Surgery, 129(6), 1277–1290.
Gimble, J. M., Grayson, W., Guilak, F., Lopez, M. J., & Vunjak-Novakovic, G. (2011). Adipose tissue as a stem cell source for musculoskeletal regeneration. Frontiers in Bioscience (Scholar Edition), 3, 69–81.
Monaco, E., Bionaz, M., Sobreira de Lima, A., Hurley, W. L., Loor, J. J., & Wheeler, M. B. (2010). Selection and reliability of internal reference genes for quantitative PCR verification of transcriptomics during the differentiation process of porcine adult mesenchymal stem cells. Stem Cell Research & Therapy, 1(1), 7.
Sági, B., Maraghechi, P., Urbán, V. S., et al. (2012). Positional identity of murine mesenchymal stem cells resident in different organs is determined in the postsegmentation mesoderm. Stem Cells and Development, 21(5), 814–828.
Sugii, S., Kida, Y., Kawamura, T., et al. (2010). Human and mouse adipose-derived cells support feeder-independent induction of pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America, 107(8), 3558–3563.
Zuk, P. A., Zhu, M., Mizuno, H., et al. (2001). Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Engineering, 7(2), 211–228.
Bourin, P., Bunnell, B. A., Casteilla, L., et al. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the international federation for adipose therapeutics and science (IFATS) and the international society for cellular therapy (ISCT). Cytotherapy, 15(6), 641–648.
Gronthos, S., & Zannettino, A. C. W. (2011). Methods for the purification and characterization of human adipose-derived stem cells. Methods in Molecular Biology (Clifton, N.J.), 702, 109–120.
Lin, G., Garcia, M., Ning, H., et al. (2008). Defining stem and progenitor cells within adipose tissue. Stem Cells and Development, 17(6), 1053–1063.
Baglioni, S., Francalanci, M., Squecco, R., et al. (2009). Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 23(10), 3494–3505.
Vishnubalaji, R., Al-Nbaheen, M., Kadalmani, B., Aldahmash, A., & Ramesh, T. (2012). Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell and Tissue Research, 347(2), 419–427.
Lin, C.-S., Xin, Z.-C., Dai, J., & Lue, T. F. (2013). Commonly used mesenchymal stem cell markers and tracking labels: limitations and challenges. Histology and Histopathology, 28(9), 1109–1116.
Sousa, B. R., Parreira, R. C., Fonseca, E. A., et al. (2014). Human adult stem cells from diverse origins: an overview from multiparametric immunophenotyping to clinical applications. Cytometry Part A: The Journal of the International Society for Analytical Cytology, 85(1), 43–77.
González-Cruz, R. D., Fonseca, V. C., & Darling, E. M. (2012). Cellular mechanical properties reflect the differentiation potential of adipose-derived mesenchymal stem cells. Proceedings of the National Academy of Sciences of the United States of America, 109(24), E1523–E1529.
Izadpanah, R., Kaushal, D., Kriedt, C., et al. (2008). Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Research, 68(11), 4229–4238.
Berdasco, M., Melguizo, C., Prados, J., et al. (2012). DNA methylation plasticity of human adipose-derived stem cells in lineage commitment. The American Journal of Pathology, 181(6), 2079–2093.
Sørensen, A. L., Timoskainen, S., West, F. D., et al. (2010). Lineage-specific promoter DNA methylation patterns segregate adult progenitor cell types. Stem Cells and Development, 19(8), 1257–1266.
Boulland, J.-L., Mastrangelopoulou, M., Boquest, A. C., et al. (2013). Epigenetic regulation of nestin expression during neurogenic differentiation of adipose tissue stem cells. Stem Cells and Development, 22(7), 1042–1052.
Kuijk, E. W., de Sousa, C., Lopes, S. M., Geijsen, N., Macklon, N., & Roelen, B. A. J. (2011). The different shades of mammalian pluripotent stem cells. Human Reproduction Update, 17(2), 254–271.
Hackett, J. A., Zylicz, J. J., & Surani, M. A. (2012). Parallel mechanisms of epigenetic reprogramming in the germline. Trends in Genetics: TIG, 28(4), 164–174.
Zhang, R., Shao, J., & Xiang, L. (2011). GADD45A protein plays an essential role in active DNA demethylation during terminal osteogenic differentiation of adipose-derived mesenchymal stem cells. The Journal of Biological Chemistry, 286(47), 41083–41094.
Samavarchi-Tehrani, P., Golipour, A., David, L., et al. (2010). Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell, 7(1), 64–77.
Seeliger, C., Culmes, M., Schyschka, L., et al. (2013). Decrease of global methylation improves significantly hepatic differentiation of Ad-MSCs: possible future application for urea detoxification. Cell Transplantation, 22(1), 119–131.
Mikkelsen, T. S., Ku, M., Jaffe, D. B., et al. (2007). Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature, 448(7153), 553–560.
Fisher, C. L., & Fisher, A. G. (2011). Chromatin states in pluripotent, differentiated, and reprogrammed cells. Current Opinion in Genetics & Development, 21(2), 140–146.
Ernst, J., Kheradpour, P., Mikkelsen, T. S., et al. (2011). Mapping and analysis of chromatin state dynamics in nine human cell types. Nature, 473(7345), 43–49.
Li, M., Liu, G.-H., & Izpisua Belmonte, J. C. (2012). Navigating the epigenetic landscape of pluripotent stem cells. Nature Reviews Molecular Cell Biology, 13(8), 524–535.
Lund, E., Oldenburg, A. R., Delbarre, E., et al. (2013). Lamin A/C-promoter interactions specify chromatin state-dependent transcription outcomes. Genome Research, 23(10), 1580–1589.
Hu, X., Fu, Y., Zhang, X., et al. (2014). Histone deacetylase inhibitor sodium butyrate promotes the osteogenic differentiation of rat adipose-derived stem cells. Development, Growth & Differentiation, 56(3), 206–213.
Noer, A., Lindeman, L. C., & Collas, P. (2009). Histone H3 modifications associated with differentiation and long-term culture of mesenchymal adipose stem cells. Stem Cells and Development, 18(5), 725–736.
Schuster-Böckler, B., & Lehner, B. (2012). Chromatin organization is a major influence on regional mutation rates in human cancer cells. Nature, 488(7412), 504–507.
Li, M. A., & He, L. (2012). microRNAs as novel regulators of stem cell pluripotency and somatic cell reprogramming. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology, 34(8), 670–680.
Ragni, E., Montemurro, T., Montelatici, E., et al. (2013). Differential microRNA signature of human mesenchymal stem cells from different sources reveals an “environmental-niche memory” for bone marrow stem cells. Experimental Cell Research, 319(10), 1562–1574.
Xu, N., Papagiannakopoulos, T., Pan, G., Thomson, J. A., & Kosik, K. S. (2009). MicroRNA-145 Regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell, 137(4), 647–658.
Adegani, F. J., Langroudi, L., Arefian, E., Shafiee, A., Dinarvand, P., & Soleimani, M. (2013). A comparison of pluripotency and differentiation status of four mesenchymal adult stem cells. Molecular Biology Reports, 40(5), 3693–3703.
Xu, C.-X., Xu, M., Tan, L., et al. (2012). MicroRNA miR-214 regulates ovarian cancer cell stemness by targeting p53/Nanog. The Journal of Biological Chemistry, 287(42), 34970–34978.
Jabbarzadeh, E., Starnes, T., Khan, Y. M., et al. (2008). Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: a combined gene therapy-cell transplantation approach. Proceedings of the National Academy of Sciences of the United States of America, 105(32), 11099–11104.
Nii, M., Lai, J. H., Keeney, M., et al. (2013). The effects of interactive mechanical and biochemical niche signaling on osteogenic differentiation of adipose-derived stem cells using combinatorial hydrogels. Acta Biomaterialia, 9(3), 5475–5483.
Uysal, C. A., Tobita, M., Hyakusoku, H., & Mizuno, H. (2014). The effect of bone-marrow-derived stem cells and adipose-derived stem cells on wound contraction and epithelization. Advances in Wound Care, 3(6), 405–413.
Tobita, M., Uysal, A. C., Ogawa, R., Hyakusoku, H., & Mizuno, H. (2008). Periodontal tissue regeneration with adipose-derived stem cells. Tissue Engineering Part A, 14(6), 945–953.
Mizuno, H., Tobita, M., & Uysal, A. C. (2012). Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells (Dayton, Ohio), 30(5), 804–810.
Chandler, E. M., Seo, B. R., Califano, J. P., et al. (2012). Implanted adipose progenitor cells as physicochemical regulators of breast cancer. Proceedings of the National Academy of Sciences of the United States of America, 109(25), 9786–9791.
Sun, N., Panetta, N. J., Gupta, D. M., et al. (2009). Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proceedings of the National Academy of Sciences of the United States of America, 106(37), 15720–15725.
Galach, M., & Utikal, J. (2011). From skin to the treatment of diseases–the possibilities of iPS cell research in dermatology. Experimental Dermatology, 20(6), 523–528.
Dudakovic, A., Camilleri, E., Riester, S. M., et al. (2014). High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. Journal of Cellular Biochemistry, 115(10), 1816–1828.
Koch, T. G., Berg, L. C., & Betts, D. H. (2009). Current and future regenerative medicine - principles, concepts, and therapeutic use of stem cell therapy and tissue engineering in equine medicine. The Canadian Veterinary Journal. La Revue Vétérinaire Canadienne, 50(2), 155–165.
Fortier, L. A., & Travis, A. J. (2011). Stem cells in veterinary medicine. Stem Cell Research & Therapy, 2(1), 9.
Wilmut, I., Schnieke, A. E., McWhir, J., Kind, A. J., & Campbell, K. H. (1997). Viable offspring derived from fetal and adult mammalian cells. Nature, 385(6619), 810–813.
Rodriguez-Osorio, N., Urrego, R., Cibelli, J. B., Eilertsen, K., & Memili, E. (2012). Reprogramming mammalian somatic cells. Theriogenology, 78(9), 1869–1886.
Kang, K.-S., & Trosko, J. E. (2011). Stem cells in toxicology: fundamental biology and practical considerations. Toxicological Sciences: An Official Journal of the Society of Toxicology, 120(Suppl 1), S269–S289.
Bilousova, G., Jun, D. H., King, K. B., et al. (2011). Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vivo. Stem Cells (Dayton, Ohio), 29(2), 206–216.
Wang, B., Miyagoe-Suzuki, Y., Yada, E., et al. (2011). Reprogramming efficiency and quality of induced pluripotent stem Cells (iPSCs) Generated from muscle-derived fibroblasts of Mdx mice at different ages. PLoS Currents, 3, RRN1274.
Yan, X., Ehnert, S., Culmes, M., et al. (2014). 5-azacytidine ımproves the osteogenic differentiation potential of aged human adipose-derived mesenchymal stem cells by DNA demethylation. PloS One, 9(6), e90846.
Lindroos, B., Aho, K.-L., Kuokkanen, H., et al. (2010). Differential gene expression in adipose stem cells cultured in allogeneic human serum versus fetal bovine serum. Tissue Engineering Part A, 16(7), 2281–2294.
Acknowledgments
FU was supported by the funding program of Research Grants for Doctoral Candidates and Young Academics and Scientists from the German Academic Exchange Service (DAAD). IDM was funded by the Undergraduate Research and Mentoring grant from the National Science Foundation and through summer research grant from the Office of Graduate Studies at Mississippi State University. ADP was supported by the National Science Foundation under award EPS 0903787. SKT was funded by the Undergraduate Research and Mentoring (URM) grant and Research Experiences for Undergraduates (REU) grant DBI-1004842 from the National Science Foundation. AMP was supported by a Research Experiences for Undergraduates (REU) grant DBI-1004842 by the National Science Foundation. Partial funding was provided by Mississippi Agricultural and Forestry Experiment Station.
Conflict of Interest
The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uzbas, F., May, I.D., Parisi, A.M. et al. Molecular Physiognomies and Applications of Adipose-Derived Stem Cells. Stem Cell Rev and Rep 11, 298–308 (2015). https://doi.org/10.1007/s12015-014-9578-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-014-9578-0