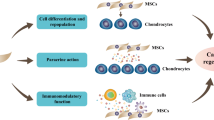
Abstract
Significant research efforts have been undertaken in the last decade in the development of stem cell-based therapies for cartilage repair. Among the various stem cell sources, mesenchymal stem cells (MSCs) demonstrate great promise and clinical efficacy in cartilage regeneration. With a deeper understanding of stem cell biology, new therapeutics and new bioengineering approaches have emerged and showed potential for further developments. Of note, there has been a paradigm shift in applying MSCs for tissue regeneration from the use of stem cells for transplantation to the use of stem cell-derived matrix and secretome components as therapeutic tools and agents for cartilage regeneration. In this review, we will discuss the emerging role of MSCs in cartilage regeneration and the most recent advances in development of stem cell-based therapeutics for cartilage regeneration.

Similar content being viewed by others
References
Hunziker, E. B. (2002). Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis and Cartilage, 10(6), 432–463.
Marcacci, M., Filardo, G., & Kon, E. (2013). Treatment of cartilage lesions: what works and why? Injury, 44(Supplement 1(0)), S11–S15.
Loeser, R. F., Goldring, S. R., Scanzello, C. R., & Goldring, M. B. (2012). Osteoarthritis: a disease of the joint as an organ. Arthritis & Rheumatism, 64(6), 1697–1707.
Ge, Z., Hu, Y., Heng, B. C., Yang, Z., Ouyang, H., Lee, E. H., et al. (2006). Osteoarthritis and therapy. Arthritis Care & Research, 55(3), 493–500.
Brittberg, M., Lindahl, A., Nilsson, A., Ohlsson, C., Isaksson, O., & Peterson, L. (1994). Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New England Journal of Medicine, 331(14), 889–895.
Jiang, Y. Z., Zhang, S. F., Qi, Y. Y., Wang, L. L., & Ouyang, H. W. (2011). Cell transplantation for articular cartilage defects: principles of past, present, and future practice. Cell Transplantation, 20(5), 593–607.
Steadman, J. R., Briggs, K. K., Rodrigo, J. J., Kocher, M. S., Gill, T. J., & Rodkey, W. G. (2003). Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 19(5), 477–484.
Revell, C. M., & Athanasiou, K. A. (2008). Success rates and immunologic responses of autogenic, allogenic, and xenogenic treatments to repair articular cartilage defects. Tissue Engineering, Part B: Reviews, 15(1), 1–15.
Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284(5411), 143–147.
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., Waknitz, M. A., Swiergiel, J. J., Marshall, V. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282(5391), 1145–1147.
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861–872.
Toh, W. S., Liu, H., Heng, B. C., Rufaihah, A. J., Ye, C. P., & Cao, T. (2005). Combined effects of TGFβ1 and BMP2 in serum-free chondrogenic differentiation of mesenchymal stem cells induced hyaline-like cartilage formation. Growth Factors, 23(4), 313–321.
Jones, B. A., & Pei, M. (2012). Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Engineering, Part B: Reviews, 18(4), 301–311.
Kuroda, R., Usas, A., Kubo, S., Corsi, K., Peng, H., Rose, T., et al. (2006). Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis & Rheumatism, 54(2), 433–442.
Nathan, S., De, S. D., Thambyah, A., Fen, C., Goh, J., & Lee, E. H. (2003). Cell-based therapy in the repair of osteochondral defects: a novel Use for adipose tissue. Tissue Engineering, 9(4), 733–744.
Huang, G. T.-J., Gronthos, S., & Shi, S. (2009). Mesenchymal stem cells derived from dental tissues vs those from other sources: their biology and role in regenerative medicine. Journal of Dental Research, 88(9), 792–806.
Nejadnik, H., Hui, J. H., Feng Choong, E. P., Tai, B.-C., & Lee, E. H. (2010). Autologous bone marrow–derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. The American Journal of Sports Medicine, 38(6), 1110–1116.
Wakitani, S., Okabe, T., Horibe, S., Mitsuoka, T., Saito, M., Koyama, T., et al. (2011). Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. Journal of Tissue Engineering and Regenerative Medicine, 5(2), 146–150.
Lian, Q., Lye, E., Suan Yeo, K., Khia Way Tan, E., Salto-Tellez, M., Liu, T. M., et al. (2007). Derivation of clinically compliant MSCs from CD105+, CD24− differentiated human ESCs. STEM CELLS, 25(2), 425–436.
Toh, W. S., Lee, E. H., & Cao, T. (2011). Potential of human embryonic stem cells in cartilage tissue engineering and regenerative medicine. Stem Cell Reviews, 7(3), 544–559.
Toh, W. S., Yang, Z., Liu, H., Heng, B. C., Lee, E. H., & Cao, T. (2007). Effects of culture conditions and bone morphogenetic protein 2 on extent of chondrogenesis from human embryonic stem cells. STEM CELLS, 25(4), 950–960.
Toh, W. S., Lee, E. H., Guo, X.-M., Chan, J. K. Y., Yeow, C. H., Choo, A. B., et al. (2010). Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials, 31(27), 6968–6980.
Ko, J.-Y., Kim, K.-I., Park, S., & Im, G.-I. (2014). In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials, 35(11), 3571–3581.
Toh, W. S., Yang, Z., Heng, B. C., & Cao, T. (2007). Differentiation of human embryonic stem cells toward the chondrogenic lineage. Methods in Molecular Biology, 407, 333–349.
da Silva, M. L., Fontes, A. M., Covas, D. T., & Caplan, A. I. (2009). Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine & Growth Factor Reviews, 20(5–6), 419–427.
Baraniak, P., & McDevitt, T. (2010). Stem cell paracrine actions and tissue regeneration. Regenerative Medicine, 5, 121–143.
Foldager, C. B., Toh, W. S., Gomoll, A. H., Olsen, B. R., & Spector, M. (2014). Distribution of basement membrane molecules, laminin and collagen type IV, in normal and degenerated cartilage tissues. Cartilage, 5, 123–132.
Kvist, A. J., Nyström, A., Hultenby, K., Sasaki, T., Talts, J. F., & Aspberg, A. (2008). The major basement membrane components localize to the chondrocyte pericellular matrix — a cartilage basement membrane equivalent? Matrix Biology, 27(1), 22–33.
Toh, W. S., Foldager, C. B., Olsen, B. R., & Spector, M. (2013). Basement membrane molecule expression attendant to chondrogenesis by nucleus pulposus cells and mesenchymal stem cells. Journal of Orthopaedic Research, 31(7), 1136–1143.
Koelling, S., Kruegel, J., Irmer, M., Path, J. R., Sadowski, B., Miro, X., et al. (2009). Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell, 4(4), 324–335.
Kon, E., Gobbi, A., Filardo, G., Delcogliano, M., Zaffagnini, S., & Marcacci, M. (2009). Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. The American Journal of Sports Medicine, 37(1), 33–41.
Scanzello, C. R., & Goldring, S. R. (2012). The role of synovitis in osteoarthritis pathogenesis. Bone, 51(2), 249–257.
Boeuf, S., & Richter, W. (2010). Chondrogenesis of mesenchymal stem cells: role of tissue source and inducing factors. Stem Cell Research & Therapy, 1(4), 31.
Liu, T. M., Martina, M., Hutmacher, D. W., Hui, J. H. P., Lee, E. H., & Lim, B. (2007). Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. STEM CELLS, 25(3), 750–760.
Afizah, H., Yang, Z., Hui, J. H. P., Ouyang, H.-W., & Lee, E.-H. (2007). A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Engineering, 13(4), 659–666.
Sakaguchi, Y., Sekiya, I., Yagishita, K., & Muneta, T. (2005). Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis & Rheumatism, 52(8), 2521–2529.
Pei, M., He, F., Boyce, B. M., & Kish, V. L. (2009). Repair of full-thickness femoral condyle cartilage defects using allogeneic synovial cell-engineered tissue constructs. Osteoarthritis and Cartilage, 17(6), 714–722.
Li, J., & Pei, M. (2012). Cell senescence: a challenge in cartilage engineering and regeneration. Tissue Engineering, Part B: Reviews, 18(4), 270–287.
Chen, X., Song, X.-H., Yin, Z., Zou, X.-H., Wang, L.-L., Hu, H., et al. (2009). Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. STEM CELLS, 27(6), 1276–1287.
Jung, Y., Bauer, G., & Nolta, J. A. (2012). Concise review: induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. STEM CELLS, 30(1), 42–47.
Villa-Diaz, L. G., Brown, S. E., Liu, Y., Ross, A. M., Lahann, J., Parent, J. M., et al. (2012). Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. STEM CELLS, 30(6), 1174–1181.
Toh, W. S., Lee, E. H., Richards, M., & Cao, T. (2010). In vitro derivation of chondrogenic cells from human embryonic stem cells. Methods in Molecular Biology, 584, 317–331.
Li, J., & Pei, M. (2010). Optimization of an in vitro three-dimensional microenvironment to reprogram synovium-derived stem cells for cartilage tissue engineering. Tissue Engineering Part A, 17(5–6), 703–712.
Hennig, T., Lorenz, H., Thiel, A., Goetzke, K., Dickhut, A., Geiger, F., et al. (2007). Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFβ receptor and BMP profile and is overcome by BMP-6. Journal of Cellular Physiology, 211(3), 682–691.
Handorf, A. M., & Li, W.-J. (2014). Induction of mesenchymal stem cell chondrogenesis through sequential administration of growth factors within specific temporal windows. Journal of Cellular Physiology, 229(2), 162–171.
Handorf, A. M., & Li, W.-J. (2011). Fibroblast growth factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis. PLoS ONE, 6(7), e22887.
Boyette, L. B., Creasey, O. A., Guzik, L., Lozito, T., & Tuan, R. S. (2014). Human bone marrow-derived mesenchymal stem cells display enhanced clonogenicity but impaired differentiation with hypoxic preconditioning. Stem Cells Translational Medicine, 3(2), 241–254.
Adesida, A., Mulet-Sierra, A., & Jomha, N. (2012). Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Research & Therapy, 3(2), 9.
Munir, S., Foldager, C., Lind, M., Zachar, V., Søballe, K., & Koch, T. (2014). Hypoxia enhances chondrogenic differentiation of human adipose tissue-derived stromal cells in scaffold-free and scaffold systems. Cell and Tissue Research, 355(1), 89–102.
Cui, J. H., Park, S. R., Park, K., Choi, B. H., & B-h, M. (2007). Preconditioning of mesenchymal stem cells with Low-intensity ultrasound for cartilage formation in vivo. Tissue Engineering, 13(2), 351–360.
Toma, C., Pittenger, M. F., Cahill, K. S., Byrne, B. J., & Kessler, P. D. (2002). Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation, 105(1), 93–98.
Tang, Y. L., Zhao, Q., Qin, X., Shen, L., Cheng, L., Ge, J., et al. (2005). Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in Rat model of myocardial infarction. The Annals of Thoracic Surgery, 80(1), 229–237.
Li, Y., Chen, J., Zhang, C. L., Wang, L., Lu, D., Katakowski, M., et al. (2005). Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia, 49(3), 407–417.
Lee, K. B. L., Hui, J. H. P., Song, I. C., Ardany, L., & Lee, E. H. (2007). Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. STEM CELLS, 25(11), 2964–2971.
Hwang, N. S., Varghese, S., Puleo, C., Zhang, Z., & Elisseeff, J. (2007). Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. Journal of Cellular Physiology, 212(2), 281–284.
Wu, L., Leijten, J. C. H., Georgi, N., Post, J. N., van Blitterswijk, C. A., & Karperien, M. (2011). Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Engineering Part A, 17(9–10), 1425–1436.
Wu, L., Prins, H.-J., Helder, M. N., van Blitterswijk, C. A., & Karperien, M. (2012). Trophic effects of mesenchymal stem cells in chondrocyte Co-cultures are independent of culture conditions and cell sources. Tissue Engineering Part A, 18(15–16), 1542–1551.
Wang, M., Rahnama, R., Cheng, T., Grotkopp, E., Jacobs, L., Limburg, S., et al. (2013). Trophic stimulation of articular chondrocytes by late-passage mesenchymal stem cells in coculture. Journal of Orthopaedic Research, 31(12), 1936–1942.
Lee, C., Burnsed, O., Raghuram, V., Kalisvaart, J., Boyan, B., & Schwartz, Z. (2012). Adipose stem cells can secrete angiogenic factors that inhibit hyaline cartilage regeneration. Stem Cell Research & Therapy, 3(4), 35.
Xu, L., Wang, Q., Xu, F., Ye, Z., Zhou, Y., & Tan, W.-S. (2013). Mesenchymal stem cells downregulate articular chondrocyte differentiation in noncontact coculture systems: implications in cartilage tissue regeneration. Stem Cells and Development, 22(11), 1657–1669.
Pei, M., Li, J., Zhang, Y., Liu, G., Wei, L., & Zhang, Y. (2014). Expansion on a matrix deposited by nonchondrogenic urine stem cells strengthens the chondrogenic capacity of repeated-passage bone marrow stromal cells. Cell and Tissue Research, 356(2), 391–403.
Jeong, S. Y., Kim, D. H., Ha, J., Jin, H. J., Kwon, S.-J., Chang, J. W., et al. (2013). Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. STEM CELLS, 31(10), 2136–2148.
Sze, S. K., de Kleijn, D. P. V., Lai, R. C., Khia Way Tan, E., Zhao, H., Yeo, K. S., et al. (2007). Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Molecular & Cellular Proteomics, 6(10), 1680–1689.
Cho, G.-W., Kang, B. Y., Kim, K.-S., & Kim, S. H. (2012). Effects of valproic acid on the expression of trophic factors in human bone marrow mesenchymal stromal cells. Neuroscience Letters, 526(2), 100–105.
Liu, G.-S., Peshavariya, H. M., Higuchi, M., Chan, E. C., Dusting, G. J., & Jiang, F. (2013). Pharmacological priming of adipose-derived stem cells for paracrine VEGF production with deferoxamine. Journal of Tissue Engineering and Regenerative Medicine. doi:10.1002/term.1796.
Lee, M. J., Kim, J., Kim, M. Y., Bae, Y.-S., Ryu, S. H., Lee, T. G., et al. (2010). Proteomic analysis of tumor necrosis factor-α-induced secretome of human adipose tissue-derived mesenchymal stem cells. Journal of Proteome Research, 9(4), 1754–1762.
Khan, M., Akhtar, S., Mohsin, S., Khan, N. S., & Riazuddin, S. (2010). Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem Cells and Development, 20(1), 67–75.
Bartosh, T. J., Ylöstalo, J. H., Mohammadipoor, A., Bazhanov, N., Coble, K., Claypool, K., et al. (2010). Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proceedings of the National Academy of Sciences, 107(31), 13724–13729.
YlÖstalo, J. H., Bartosh, T. J., Coble, K., & Prockop, D. J. (2012). Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. STEM CELLS, 30(10), 2283–2296.
Bara, J. J., McCarthy, H. E., Humphrey, E., Johnson, W. E. B., & Roberts, S. (2013). Bone marrow-derived mesenchymal stem cells become antiangiogenic when chondrogenically or osteogenically differentiated: implications for bone and cartilage tissue engineering. Tissue Engineering Part A, 20(1–2), 147–159.
Kubo, S., Cooper, G., Matsumoto, T., Phillippi, J., Corsi, K., Usas, A., et al. (2009). Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle-derived stem cells. Arthritis and Rheumatism, 60, 155–165.
Petrie Aronin, C. E., & Tuan, R. S. (2010). Therapeutic potential of the immunomodulatory activities of adult mesenchymal stem cells. Birth Defects Research Part C: Embryo Today: Reviews, 90(1), 67–74.
Tetta, C., Bruno, S., Fonsato, V., Deregibus, M. C., & Camussi, G. (2011). The role of microvesicles in tissue repair. Organogenesis, 7(2), 105–115.
Yeo, R. W. Y., Lai, R. C., Zhang, B., Tan, S. S., Yin, Y., Teh, B. J., et al. (2013). Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Advanced Drug Delivery Reviews, 65(3), 336–341.
Goldring, M., Tsuchimochi, K., & Ijiri, K. (2006). The control of chondrogenesis. Journal of Cellular Biochemistry, 97, 33–44.
Huang, Q., Goh, J. C. H., Hutmacher, D. W., & Lee, E. H. (2002). In vivo mesenchymal cell recruitment by a scaffold loaded with transforming growth factor β1 and the potential for in situ chondrogenesis. Tissue Engineering, 8(3), 469–482.
Gaissmaier, C., Koh, J. L., & Weise, K. (2008). Growth and differentiation factors for cartilage healing and repair. Injury, 39(1), 88–96.
Schmidt, M. B., Chen, E. H., & Lynch, S. E. (2006). A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthritis and Cartilage, 14(5), 403–412.
Zhang, W., Chen, J., Tao, J., Jiang, Y., Hu, C., Huang, L., et al. (2013). The use of type 1 collagen scaffold containing stromal cell-derived factor-1 to create a matrix environment conducive to partial-thickness cartilage defects repair. Biomaterials, 34(3), 713–723.
Liu, H., Lu, K., MacAry, P. A., Wong, K. L., Heng, A., Cao, T., et al. (2012). Soluble molecules are key in maintaining the immunomodulatory activity of murine mesenchymal stromal cells. Journal of Cell Science, 125(1), 200–208.
Manferdini, C., Maumus, M., Gabusi, E., Piacentini, A., Filardo, G., Peyrafitte, J.-A., et al. (2013). Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis & Rheumatism, 65(5), 1271–1281.
Patra, D., & Sandell, L. J. (2012). Antiangiogenic and anticancer molecules in cartilage. Expert Reviews in Molecular Medicine, 14, e10.
Fu, X., Toh, W. S., Liu, H., Lu, K., Li, M., & Cao, T. (2011). Establishment of clinically compliant human embryonic stem cells in an autologous feeder-free system. Tissue Engineering. Part C, Methods, 17(9), 927–937.
Peng, Y., Bocker, M. T., Holm, J., Toh, W. S., Hughes, C. S., Kidwai, F., et al. (2012). Human fibroblast matrices bio-assembled under macromolecular crowding support stable propagation of human embryonic stem cells. Journal of Tissue Engineering and Regenerative Medicine, 6(10), e74–e86.
Sun, Y., Li, W., Lu, Z., Chen, R., Ling, J., Ran, Q., et al. (2011). Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. The FASEB Journal, 25(5), 1474–1485.
Pei, M., He, F., Li, J., Tidwell, J. E., Jones, A. C., & McDonough, E. B. (2012). Repair of large animal partial-thickness cartilage defects through intraarticular injection of matrix-rejuvenated synovium-derived stem cells. Tissue Engineering Part A, 19(9–10), 1144–1154.
He, F., Chen, X., & Pei, M. (2009). Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering. Tissue Engineering Part A, 15(12), 3809–3821.
Pei, M., & He, F. (2012). Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. Journal of Cellular Physiology, 227(5), 2163–2174.
Pei, M., Shoukry, M., Li, J., Daffner, S. D., France, J. C., & Emery, S. E. (2012). Modulation of in vitro microenvironment facilitates synovium-derived stem cell-based nucleus pulposus tissue regeneration. Spine, 37(18), 1538–1547.
He, F. P., & Pei, M. (2012). Rejuvenation of nucleus pulposus cells using extracellular matrix deposited by synovium-derived stem cells. Spine, 37(6), 459–469.
Pei, M., Zhang, Y., Li, J., & Chen, D. (2012). Antioxidation of decellularized stem cell matrix promotes human synovium-derived stem cell-based chondrogenesis. Stem Cells and Development, 22(6), 889–900.
Pei, M., He, F., & Kish, V. L. (2011). Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Engineering Part A, 17(23–24), 3067–3076.
Gilbert, T. W., Sellaro, T. L., & Badylak, S. F. (2006). Decellularization of tissues and organs. Biomaterials, 27(19), 3675–3683.
Chen, C., Loe, F., Blocki, A., Peng, Y., & Raghunath, M. (2011). Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies. Advanced Drug Delivery Reviews, 63(4–5), 277–290.
Lindner, U., Kramer, J., Behrends, J., Driller, B., Wendler, N.-O., Boehrnsen, F., et al. (2010). Improved proliferation and differentiation capacity of human mesenchymal stromal cells cultured with basement-membrane extracellular matrix proteins. Cytotherapy, 12(8), 992–1005.
Helledie, T., Dombrowski, C., Rai, B., Lim, Z. X. H., Hin, I. L. H., Rider, D. A., et al. (2011). Heparan sulfate enhances the self-renewal and therapeutic potential of mesenchymal stem cells from human adult bone marrow. Stem Cells and Development, 21(11), 1897–1910.
Li, J., Hansen, K. C., Zhang, Y., Dong, C., Dinu, C. Z., Dzieciatkowska, M., et al. (2014). Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials, 35(2), 642–653.
Toh, W. S., & Loh, X. J. (2014). Advances in hydrogel delivery systems for tissue regeneration. Materials Science and Engineering: C. doi:10.1016/j.msec.2014.04.026.
Seib, F. P., Prewitz, M., Werner, C., & Bornhäuser, M. (2009). Matrix elasticity regulates the secretory profile of human bone marrow-derived multipotent mesenchymal stromal cells (MSCs). Biochemical and Biophysical Research Communications, 389(4), 663–667.
Toh, W. S., Spector, M., Lee, E. H., & Cao, T. (2011). Biomaterial-mediated delivery of microenvironmental cues for repair and regeneration of articular cartilage. Molecular Pharmaceutics, 8(4), 994–1001.
He, J., Genetos, D. C., & Leach, J. K. (2009). Osteogenesis and trophic factor secretion are influenced by the composition of hydroxyapatite/poly(lactide-Co-glycolide) composite scaffolds. Tissue Engineering Part A, 16(1), 127–137.
Bosnakovski, D., Mizuno, M., Kim, G., Takagi, S., Okumura, M., & Fujinaga, T. (2006). Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnology and Bioengineering, 93(6), 1152–1163.
Toh, W. S., Guo, X.-M., Choo, A. B., Lu, K., Lee, E. H., & Cao, T. (2009). Differentiation and enrichment of expandable chondrogenic cells from human embryonic stem cells in vitro. Journal of Cellular and Molecular Medicine, 13(9b), 3570–3590.
Toh, W. S., Lim, T. C., Kurisawa, M., & Spector, M. (2012). Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials, 33(15), 3835–3845.
Wu, S.-C., Chang, J.-K., Wang, C.-K., Wang, G.-J., & Ho, M.-L. (2010). Enhancement of chondrogenesis of human adipose derived stem cells in a hyaluronan-enriched microenvironment. Biomaterials, 31(4), 631–640.
Jose, S., Hughbanks, M. L., Binder, B. Y. K., Ingavle, G. C., & Leach, J. K. (2014). Enhanced trophic factor secretion by mesenchymal stem/stromal cells with Glycine-Histidine-Lysine (GHK)-modified alginate hydrogels. Acta Biomaterialia, 10(5), 1955–1964.
Silva, N. A., Moreira, J., Ribeiro-Samy, S., Gomes, E. D., Tam, R. Y., Shoichet, M. S., et al. (2013). Modulation of bone marrow mesenchymal stem cell secretome by ECM-like hydrogels. Biochimie, 95(12), 2314–2319.
Schwarz, S., Koerber, L., Elsaesser, A. F., Goldberg-Bockhorn, E., Seitz, A. M., Dürselen, L., et al. (2012). Decellularized cartilage matrix as a novel biomatrix for cartilage tissue-engineering applications. Tissue Engineering Part A, 18(21–22), 2195–2209.
Lu, Q., Li, M., Zou, Y., & Cao, T. (2014). Delivery of basic fibroblast growth factors from heparinized decellularized adipose tissue stimulates potent de novo adipogenesis. Journal of Controlled Release, 174, 43–50.
Cheung, H. K., Han, T. T. Y., Marecak, D. M., Watkins, J. F., Amsden, B. G., & Flynn, L. E. (2014). Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials, 35(6), 1914–1923.
Sawkins, M. J., Bowen, W., Dhadda, P., Markides, H., Sidney, L. E., Taylor, A. J., et al. (2013). Hydrogels derived from demineralized and decellularized bone extracellular matrix. Acta Biomaterialia, 9(8), 7865–7873.
Adam Young, D., Bajaj, V., & Christman, K. L. (2014). Decellularized adipose matrix hydrogels stimulate in vivo neovascularization and adipose formation. Journal of Biomedical Materials Research, Part A, 102(6), 1641–1651.
Li, W.-J., Tuli, R., Huang, X., Laquerriere, P., & Tuan, R. S. (2005). Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials, 26(25), 5158–5166.
Garrigues, N. W., Little, D., Sanchez-Adams, J., Ruch, D. S., & Guilak, F. (2014). Electrospun cartilage-derived matrix scaffolds for cartilage tissue engineering. Journal of Biomedical Materials Research, Part A. doi:10.1002/jbm.a.35068.
Loh, X. J., Peh, P., Liao, S., Sng, C., & Li, J. (2010). Controlled drug release from biodegradable thermoresponsive physical hydrogel nanofibers. Journal of Controlled Release, 143(2), 175–182.
Lim, T. C., Rokkappanavar, S., Toh, W. S., Wang, L.-S., Kurisawa, M., & Spector, M. (2013). Chemotactic recruitment of adult neural progenitor cells into multifunctional hydrogels providing sustained SDF-1α release and compatible structural support. The FASEB Journal, 27(3), 1023–1033.
Diao, H. J., Yeung, C. W., Yan, C. H., Chan, G. C. F., & Chan, B. P. (2013). Bidirectional and mutually beneficial interactions between human mesenchymal stem cells and osteoarthritic chondrocytes in micromass co-cultures. Regenerative Medicine, 8(3), 257–269.
Acknowledgments
This work was partially supported by grants (R221000068720 and R221000070733) from National University of Singapore, National University Healthcare System, and Ministry of Education, Singapore.
Disclosure
The authors indicate no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toh, W.S., Foldager, C.B., Pei, M. et al. Advances in Mesenchymal Stem Cell-based Strategies for Cartilage Repair and Regeneration. Stem Cell Rev and Rep 10, 686–696 (2014). https://doi.org/10.1007/s12015-014-9526-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-014-9526-z