Abstract
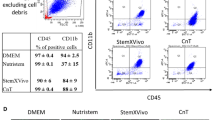
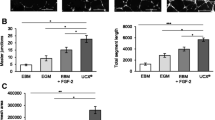
Peripheral vascular disease affects ~20 % of the population over 50 years of age and is a complication of type 2 diabetes. Cell therapy studies revealed that cells from older or diabetic donors have a reduced capacity to induce tissue repair compared to healthy and younger cells. This fact greatly impedes the use of autologous cells for treatment. Umbilical cord blood CD34+ cells are a source of angiogenic cells but unlike bone marrow CD34+ angiogenic cells, achieving clinically significant cell numbers has been difficult without in vitro expansion. We report here that culturing CD34+/CD45+ blood cells from frozen umbilical cord blood units in a medium supplemented with FGF4, SCF and FLT3-ligand produced a population of cells that remain CD34+/CD45+ but have an increased capacity for tissue healing. The cultured CD34+ cells were compared directly to non-cultured CD34+ cells in a mouse model of ischemia. Cultured CD34+ cells demonstrated strong paracrine signaling as well as the capacity to differentiate into endothelial cells, smooth muscle and striated muscle. We observed an improvement in blood flow and a significant reduction in foot necrosis. A second study was completed to assess the safety of the cells. No adverse effects were associated with the injection of the cultured cells. Our method described here for culturing umbilical cord blood cells resulted in cells with a strong paracrine effect that induces substantial tissue repair in a murine model of hind limb ischemia and evidence of engraftment and differentiation of the cultured cells into new vasculature and muscle.






Similar content being viewed by others
References
Staudacher, D. L., Preis, M., Lewis, B. S., Grossman, P. M., & Flugelman, M. Y. (2006). Cellular and molecular therapeutic modalities for arterial obstructive syndromes. Pharmacology and Therapeutics, 109, 263–273.
Rice, T. W., & Lumsden, A. B. (2006). Optimal medical management of peripheral arterial disease. Vascular and Endovascular Surgery, 40, 312–327.
Feener, E. P., & King, G. L. (1997). Vascular dysfunction in diabetes mellitus. Lancet, 350(Suppl 1), SI9–SI13.
Copland, I., Sharma, K., Lejeune, L., et al. (2008). CD34 expression on murine marrow-derived mesenchymal stromal cells: impact on neovascularization. Experimental Hematology, 36, 93–103.
Idei, N., Soga, J., Hata, T., et al. (2011). Autologous bone-marrow mononuclear cell implantation reduces long-term major amputation risk in patients with critical limb ischemia: a comparison of atherosclerotic peripheral arterial disease and Buerger disease. Circulation. Cardiovascular Interventions, 4, 15–25.
Jujo, K., Ii, M., & Losordo, D. W. (2008). Endothelial progenitor cells in neovascularization of infarcted myocardium. Journal of Molecular and Cellular Cardiology, 45, 530–544.
Elsharawy, M. A., Naim, M., & Greish, S. (2012). Human CD34+ stem cells promote healing of diabetic foot ulcers in rats. Interactive Cardiovascular and Thoracic Surgery, 14, 288–293.
Kawamoto, A., Katayama, M., Handa, N., et al. (2009). Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells, 27, 2857–2864.
Zhuo, Y., Li, S. H., Chen, M. S., et al. (2010). Aging impairs the angiogenic response to ischemic injury and the activity of implanted cells: combined consequences for cell therapy in older recipients. The Journal of Thoracic and Cardiovascular Surgery, 139, 1286–1294. 94 e1-2.
Sugihara, S., Yamamoto, Y., Matsuura, T., et al. (2007). Age-related BM-MNC dysfunction hampers neovascularization. Mechanisms of Ageing and Development, 128, 511–516.
Smadja, D. M., Duong-van-Huyen, J. P., Dal Cortivo, L., et al. (2012). Early endothelial progenitor cells in bone marrow are a biomarker of cell therapy success in patients with critical limb ischemia. Cytotherapy, 14, 232–239.
Caballero, S., Sengupta, N., Afzal, A., et al. (2007). Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes, 56, 960–967.
Fadini, G. P., Miorin, M., Facco, M., et al. (2005). Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. Journal of the American College of Cardiology, 45, 1449–1457.
Vanneaux, V., El-Ayoubi, F., Delmau, C., et al. (2010). In vitro and in vivo analysis of endothelial progenitor cells from cryopreserved umbilical cord blood: are we ready for clinical application? Cell Transplantation, 19, 1143–1155.
Barclay, G. R., Tura, O., Samuel, K., et al. (2012). Systematic assessment in an animal model of the angiogenic potential of different human cell sources for therapeutic revascularization. Stem Cell Research & Therapy, 3, 23.
Mareschi, K., Biasin, E., Piacibello, W., Aglietta, M., Madon, E., & Fagioli, F. (2001). Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica, 86, 1099–1100.
Erices, A., Conget, P., & Minguell, J. J. (2000). Mesenchymal progenitor cells in human umbilical cord blood. British Journal of Haematology, 109, 235–242.
Kern, S., Eichler, H., Stoeve, J., Kluter, H., & Bieback, K. (2006). Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood or adipose tissue. Stem Cells, 24, 1294–1301.
Bieback, K., Kern, S., Kluter, H., & Eichler, H. (2004). Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells, 22, 625–634.
Rogers, I., Yamanaka, N., Bielecki, R., et al. (2007). Identification and analysis of in vitro cultured CD45-positive cells capable of multi-lineage differentiation. Experimental Cell Research, 313, 1839–1852.
Rogers, I. M., Yamanaka, N., & Casper, R. F. (2008). A simplified procedure for hematopoietic stem cell amplification using a serum-free, feeder cell-free culture system. Biology of Blood and Marrow Transplantation: Journal of the American Society for Blood and Marrow Transplantation, 14, 927–937.
Wong, C. J., Casper, R. F., & Rogers, I. M. (2010). Epigenetic changes to human umbilical cord blood cells cultured with three proteins indicate partial reprogramming to a pluripotent state. Experimental Cell Research, 316, 927–939.
Chua, S. J., Bielecki, R., Yamanaka, N., Fehlings, M. G., Rogers, I. M., & Casper, R. F. (2010). The effect of umbilical cord blood cells on outcomes after experimental traumatic spinal cord injury. Spine (Phila Pa 1976), 35, 1520–1526.
Helisch, A., Wagner, S., Khan, N., et al. (2006). Impact of mouse strain differences in innate hindlimb collateral vasculature. Arteriosclerosis, Thrombosis, and Vascular Biology, 26, 520–526.
Walter, D. H., Krankenberg, H., Balzer, J. O., et al. (2011). Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA). Circulation. Cardiovascular Interventions, 4, 26–37.
Shultz, L. D., Schweitzer, P. A., Christianson, S. W., et al. (1995). Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. Journal of Immunology, 154, 180–191.
Sonnemann, K. J., & Bement, W. M. (2011). Wound repair: toward understanding and integration of single-cell and multicellular wound responses. Annual Review of Cell and Developmental Biology, 27, 237–263.
Sivan-Loukianova, E., Awad, O. A., Stepanovic, V., Bickenbach, J., & Schatteman, G. C. (2003). CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. Journal of Vascular Research, 40, 368–377.
Ward, M. R., Stewart, D. J., & Kutryk, M. J. (2007). Endothelial progenitor cell therapy for the treatment of coronary disease, acute MI, and pulmonary arterial hypertension: current perspectives. Catheterization and Cardiovascular Interventions, 70, 983–998.
Akiyama, K., You, Y. O., Yamaza, T., et al. (2012). Characterization of bone marrow derived mesenchymal stem cells in suspension. Stem Cell Research & Therapy, 3, 40.
Lin, C. S., Ning, H., Lin, G., & Lue, T. F. (2012). Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy, 14, 1159–1163.
Lian, Q., Zhang, Y., Zhang, J., et al. (2010). Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation, 121, 1113–1123.
Fadini, G. P., Sartore, S., Albiero, M., et al. (2006). Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arteriosclerosis, Thrombosis, and Vascular Biology, 26, 2140–2146.
Klibansky, D. A., Chin, A., Duignan, I. J., & Edelberg, J. M. (2006). Synergistic targeting with bone marrow-derived cells and PDGF improves diabetic vascular function. American Journal of Physiology. Heart and Circulatory Physiology, 290, H1387–H1392.
Qiang, T., Lugui, Q., Guangping, L., Changhong, L., Chenghuan, Z., Hengxing, M., Wansong, Y., (2010). Transplantation of healthy but not diabetic outgrowth endothelial cells could rescue ischemic myocardium in diabetic rabbits. Scandinavian Journal of Clinical & Laboratory Investigation.
Schatteman, G. C., Hanlon, H. D., Jiao, C., Dodds, S. G., & Christy, B. A. (2000). Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. Journal of Clinical Investigation, 106, 571–578.
Gupta, R., & Losordo, D. W. (2011). Cell therapy for critical limb ischemia: moving forward one step at a time. Circulation. Cardiovascular Interventions, 4, 2–5.
Acknowledgments
Umbilical Cord Blood Collections: Research Centre for Women’s and Infants’ Health, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Canada, http://biobank.lunenfeld.ca
Funding
CIHR POP grant, Canadian Stem Cell Network, MaRS Innovations Proof of Principle Grant, CFI LEF Project 20694, Insception-Life Banks.
Conflict of Interest Disclosures
IMR, RFC are founders of Insception Biosciences.
Author Contributions
Jennifer Whiteley: Collection/assembly of data, data analysis/interpretation, manuscript writing
Ryszard Bielecki: Collection/assembly of data, data analysis/interpretation, manuscript writing
Mira Li: Collection/assembly of data, data analysis/interpretation, manuscript writing
Shawn Chua: Collection/assembly of data
Michael R. Ward: Collection/assembly of data, manuscript writing
Nobuko Yamanaka: Collection/assembly of data, data analysis/interpretation, manuscript writing
Duncan J. Stewart: Concept and design, financial support, data analysis and interpretation, manuscript writing
Robert F. Casper: Financial support, manuscript writing
Ian M. Rogers: Concept and design, financial support, administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Whiteley, J., Bielecki, R., Li, M. et al. An Expanded Population of CD34+ Cells from Frozen Banked Umbilical Cord Blood Demonstrate Tissue Repair Mechanisms of Mesenchymal Stromal Cells and Circulating Angiogenic Cells in an Ischemic Hind Limb Model. Stem Cell Rev and Rep 10, 338–350 (2014). https://doi.org/10.1007/s12015-014-9496-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-014-9496-1