Abstract
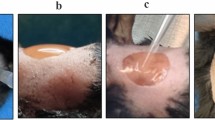
Numerous studies have shown the presence of high levels of growth factors during the process of healing. Growth factors act by binding to the cell surface receptors and contribute to the subsequent activation of signal transduction mechanisms. Wound healing requires a complex of biological and molecular events that includes attraction and proliferation of different type of cells to the wound site, differentiation and angiogenesis. More specifically, migration of various cell types, such as endothelial cells and their precursors, mesenchymal stem/stromal cells (MSCs) or skin fibroblasts (DFs) plays an important role in the healing process. In recent years, the application of platelet rich plasma (PRP) to surgical wounds and skin ulcerations is becoming more frequent, as it is believed to accelerate the healing process. The local enrichment of growth factors at the wound after PRP application causes a stimulation of tissue regeneration. Herein, we studied: (i) the effect of autologous PRP in skin ulcers of patients of different aetiology, (ii) the proteomic profile of PRP, (iii) the migration potential of amniotic fluid MSCs and DFs in the presence of PRP extract in vitro, (iv) the use of the PRP extract as a substitute for serum in cultivating AF-MSCs. Considering its easy access, PRP may provide a valuable tool in multiple therapeutic approaches.




Similar content being viewed by others
References
Abdulrazzak, H., Moschidou, D., Jones, G., & Guillot, P. V. (2010). Biological characteristics of stem cells from foetal, cord blood and extraembryonic tissues. Journal of the Royal Society Interface, 7(Suppl 6), S689–706.
Abiko, Y., Arai, J., Matsuzawa, K., Inoue, T., Shimono, M., & Kaku, T. (1996). Human gingival fibroblast migration promoted by platelet-derived growth factor on titanium is correlated with release of urokinase type plasminogen activator. The Bulletin of Tokyo Dental College, 37, 113–118.
Amable, P. R., Carias, R. B., Teixeira, M. V., da Cruz Pacheco, I., Correa do Amaral, R. J., Granjeiro, J. M., & Borojevic, R. (2013). Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Research & Therapy, 4, 67.
Anitua, E., Muruzabal, F., Alcalde, I., Merayo-Lloves, J. & Orive, G. (2013) Plasma Rich in Growth Factors (PRGF-Endoret) stimulates corneal wound healing and reduces haze formation after PRK surgery. Exp Eye Res
Bitsika, V., Roubelakis, M.G., Zagoura, D., Trohatou, O., Makridakis, M., Pappa, K.I., Marini, F.C., Vlahou, A. & Anagnou, N.P. (2011) Human Amniotic Fluid-Derived Mesenchymal Stem Cells As Therapeutic Vehicles: A Novel Approach For the Treatment of Bladder Cancer. Stem Cells Dev
Bitsika, V., Vlahou, A., & Roubelakis, M. G. (2013). Fetal mesenchymal stem cells in cancer therapy. Current Stem Cell Research & Therapy, 8, 133–143.
Blume, A., Berger, M., Benie, A. J., Peters, T., & Hinderlich, S. (2008). Characterization of ligand binding to N-acetylglucosamine kinase studied by STD NMR. Biochemistry, 47, 13138–13146.
Brem, H., Balledux, J., Bloom, T., Kerstein, M. D., & Hollier, L. (2000). Healing of diabetic foot ulcers and pressure ulcers with human skin equivalent: a new paradigm in wound healing. Archives of Surgery, 135, 627–634.
Bruno, S., Grange, C., Deregibus, M. C., Calogero, R. A., Saviozzi, S., Collino, F., Morando, L., Busca, A., Falda, M., Bussolati, B., Tetta, C., & Camussi, G. (2009). Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. Journal of the American Society of Nephrology, 20, 1053–1067.
Campagnoli, C., Bellantuono, I., Kumar, S., Fairbairn, L. J., Roberts, I., & Fisk, N. M. (2002). High transduction efficiency of circulating first trimester fetal mesenchymal stem cells: potential targets for in utero ex vivo gene therapy. BJOG, 109, 952–954.
De Coppi, P., Bartsch, G., Jr., Siddiqui, M. M., Xu, T., Santos, C. C., Perin, L., Mostoslavsky, G., Serre, A. C., Snyder, E. Y., Yoo, J. J., Furth, M. E., Soker, S., & Atala, A. (2007). Isolation of amniotic stem cell lines with potential for therapy. Nature Biotechnology, 25, 100–106.
Delorme, B., Ringe, J., Pontikoglou, C., Gaillard, J., Langonne, A., Sensebe, L., Noel, D., Jorgensen, C., Haupl, T., & Charbord, P. (2009). Specific lineage-priming of bone marrow mesenchymal stem cells provides the molecular framework for their plasticity. Stem Cells, 27, 1142–1151.
Doucet, C., Ernou, I., Zhang, Y., Llense, J. R., Begot, L., Holy, X., & Lataillade, J. J. (2005). Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. Journal of Cellular Physiology, 205, 228–236.
Driver, V. R., Hanft, J., Fylling, C. P., & Beriou, J. M. (2006). A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy/Wound Management, 52, 68–70. 72, 74 passim.
Dufour, A., Zucker, S., Sampson, N. S., Kuscu, C., & Cao, J. (2010). Role of matrix metalloproteinase-9 dimers in cell migration: design of inhibitory peptides. Journal of Biological Chemistry, 285, 35944–35956.
English, K., French, A., & Wood, K. J. (2011). Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell, 7, 431–442.
Falanga, V. (2005). Wound healing and its impairment in the diabetic foot. Lancet, 366, 1736–1743.
Freinkel, R. K., & Woodley, D. T. (2001). The Biology of Skin. New York, London: The Parthenon Publishing Group.
Gazouli, M., Roubelakis, M. G., Theodoropoulos, G. E., Papailiou, J., Vaiopoulou, A., Pappa, K. I., Nikiteas, N., & Anagnou, N. P. (2011). OCT4 spliced variant OCT4B1 is expressed in human colorectal cancer. Molecular Carcinogenesis, 51, 165–173.
Hocking, A. M., & Gibran, N. S. (2010). Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Experimental Cell Research, 316, 2213–2219.
In’t Anker, P. S., Scherjon, S. A., Kleijburg-van der Keur, C., Noort, W. A., Claas, F. H., Willemze, R., Fibbe, W. E., & Kanhai, H. H. (2003). Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood, 102, 1548–1549.
Javazon, E. H., Keswani, S. G., Badillo, A. T., Crombleholme, T. M., Zoltick, P. W., Radu, A. P., Kozin, E. D., Beggs, K., Malik, A. A., & Flake, A. W. (2007). Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair and Regeneration, 15, 350–359.
Kim, J. Y., Song, S. H., Kim, K. L., Ko, J. J., Im, J. E., Yie, S. W., Ahn, Y. K., Kim, D. K., & Suh, W. (2010). Human cord blood-derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing. Cell Transplantation, 19, 1635–1644.
Klemmt, P. A., Vafaizadeh, V., & Groner, B. (2011). The potential of amniotic fluid stem cells for cellular therapy and tissue engineering. Expert Opinion on Biological Therapy, 11, 1297–1314.
Knighton, D. R., Ciresi, K., Fiegel, V. D., Schumerth, S., Butler, E., & Cerra, F. (1990). Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet-derived wound healing formula. Surgery, Gynecology & Obstetrics, 170, 56–60.
Kuo, Y. R., Wang, C. T., Cheng, J. T., Wang, F. S., Chiang, Y. C., & Wang, C. J. (2011). Bone marrow-derived mesenchymal stem cells enhanced diabetic wound healing through recruitment of tissue regeneration in a rat model of streptozotocin-induced diabetes. Plastic and Reconstructive Surgery, 128, 872–880.
Kwon, D. S., Gao, X., Liu, Y. B., Dulchavsky, D. S., Danyluk, A. L., Bansal, M., Chopp, M., McIntosh, K., Arbab, A. S., Dulchavsky, S. A., & Gautam, S. C. (2008). Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. International Wound Journal, 5, 453–463.
Lin, C. D., Allori, A. C., Macklin, J. E., Sailon, A. M., Tanaka, R., Levine, J. P., Saadeh, P. B., & Warren, S. M. (2008). Topical lineage-negative progenitor-cell therapy for diabetic wounds. Plastic and Reconstructive Surgery, 122, 1341–1351.
Makridakis, M., Gagos, S., Petrolekas, A., Roubelakis, M. G., Bitsika, V., Stravodimos, K., Pavlakis, K., Anagnou, N. P., Coleman, J., & Vlahou, A. (2009). Chromosomal and proteome analysis of a new T24-based cell line model for aggressive bladder cancer. Proteomics, 9, 287–298.
Marrotte, E. J., Chen, D. D., Hakim, J. S., & Chen, A. F. (2010). Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. Journal of Clinical Investigation, 120, 4207–4219.
Martin-Rendon, E., Sweeney, D., Lu, F., Girdlestone, J., Navarrete, C., & Watt, S. M. (2008). 5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox Sanguinis, 95, 137–148.
Martin, P. (1997). Wound healing–aiming for perfect skin regeneration. Science, 276, 75–81.
Martinez-Zapata, M. J., Marti-Carvajal, A., Sola, I., Bolibar, I., Angel Exposito, J., Rodriguez, L., & Garcia, J. (2009). Efficacy and safety of the use of autologous plasma rich in platelets for tissue regeneration: a systematic review. Transfusion, 49, 44–56.
Mazzucco, L., Medici, D., Serra, M., Panizza, R., Rivara, G., Orecchia, S., Libener, R., Cattana, E., Levis, A., Betta, P. G., & Borzini, P. (2004). The use of autologous platelet gel to treat difficult-to-heal wounds: a pilot study. Transfusion, 44, 1013–1018.
McAleer, J. P., Sharma, S., Kaplan, E. M., & Persich, G. (2006). Use of autologous platelet concentrate in a nonhealing lower extremity wound. Advances in Skin & Wound Care, 19, 354–363.
Mirabella, T., Hartinger, J., Lorandi, C., Gentili, C., van Griensven, M., & Cancedda, R. (2012). Proangiogenic soluble factors from amniotic fluid stem cells mediate the recruitment of endothelial progenitors in a model of ischemic fasciocutaneous flap. Stem Cells and Development, 21, 2179–2188.
Morigi, M., Rota, C., Montemurro, T., Montelatici, E., Lo Cicero, V., Imberti, B., Abbate, M., Zoja, C., Cassis, P., Longaretti, L., Rebulla, P., Introna, M., Capelli, C., Benigni, A., Remuzzi, G., & Lazzari, L. (2011). Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cells, 28, 513–522.
Mosna, F., Sensebe, L., & Krampera, M. (2011). Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells and Development, 19, 1449–1470.
Murphy, M. B., Blashki, D., Buchanan, R. M., Yazdi, I. K., Ferrari, M., Simmons, P. J., & Tasciotti, E. (2012). Adult and umbilical cord blood-derived platelet-rich plasma for mesenchymal stem cell proliferation, chemotaxis, and cryo-preservation. Biomaterials, 33, 5308–5316.
Prusa, A. R., Marton, E., Rosner, M., Bernaschek, G., & Hengstschlager, M. (2003). Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Human Reproduction, 18, 1489–1493.
Roubelakis, M. G. (2013). Therapeutic potential of fetal mesenchymal stem cells. Current Stem Cell Research & Therapy, 8, 115–116.
Roubelakis, M. G., Bitsika, V., Zagoura, D., Trohatou, O., Pappa, K. I., Makridakis, M., Antsaklis, A., Vlahou, A., & Anagnou, N. P. (2010). In vitro and in vivo properties of distinct populations of amniotic fluid mesenchymal progenitor cells. Journal of Cellular and Molecular Medicine, 15, 1896–1913.
Roubelakis, M. G., Pappa, K. I., Bitsika, V., Zagoura, D., Vlahou, A., Papadaki, H. A., Antsaklis, A., & Anagnou, N. P. (2007). Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells and Development, 16, 931–952.
Roubelakis, M. G., Trohatou, O., & Anagnou, N. P. (2012). Amniotic fluid and amniotic membrane stem cells: marker discovery. Stem Cells International, 2012, 107836.
Roubelakis, M. G., Tsaknakis, G., Pappa, K. I., Anagnou, N. P., & Watt, S. M. (2013). Spindle shaped human mesenchymal stem/stromal cells from amniotic fluid promote neovascularization. PLoS One, 8, e54747.
Saldalamacchia, G., Lapice, E., Cuomo, V., De Feo, E., D’Agostino, E., Rivellese, A. A., & Vaccaro, O. (2004). A controlled study of the use of autologous platelet gel for the treatment of diabetic foot ulcers. Nutrition, Metabolism, and Cardiovascular Diseases, 14, 395–396.
Schmidt, B. A., & Horsley, V. (2013). Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development, 140, 1517–1527.
Singer, A. J., & Clark, R. A. (1999). Cutaneous wound healing. New England Journal of Medicine, 341, 738–746.
Trohatou, O., Anagnou, N. P., & Roubelakis, M. G. (2013). Human amniotic fluid stem cells as an attractive tool for clinical applications. Current Stem Cell Research & Therapy, 8, 125–132.
Troyer, D. L., & Weiss, M. L. (2008). Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells, 26, 591–599.
Tsai, M. S., Hwang, S. M., Tsai, Y. L., Cheng, F. C., Lee, J. L., & Chang, Y. J. (2006). Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells. Biology of Reproduction, 74, 545–551.
Tsai, M. S., Lee, J. L., Chang, Y. J., & Hwang, S. M. (2004). Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Human Reproduction, 19, 1450–1456.
Tzeng, Y. S., Deng, S. C., Wang, C. H., Tsai, J. C., Chen, T. M., & Burnouf, T. (2013). Treatment of nonhealing diabetic lower extremity ulcers with skin graft and autologous platelet gel: a case series. BioMed Research International, 2013, 837620.
van den Dolder, J., Mooren, R., Vloon, A. P., Stoelinga, P. J., & Jansen, J. A. (2006). Platelet-rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Engineering, 12, 3067–3073.
Volarevic, V., Arsenijevic, N., Lukic, M. L., & Stojkovic, M. (2011). Concise review: Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells, 29, 5–10.
Wang, L., Zhang, Z. G., Zhang, R. L., Gregg, S. R., Hozeska-Solgot, A., LeTourneau, Y., Wang, Y., & Chopp, M. (2006). Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. Journal of Neuroscience, 26, 5996–6003.
Wu, Y., Chen, L., Scott, P. G., & Tredget, E. E. (2007). Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells, 25, 2648–2659.
Yoon, B. S., Moon, J. H., Jun, E. K., Kim, J., Maeng, I., Kim, J. S., Lee, J. H., Baik, C. S., Kim, A., Cho, K. S., Lee, H. H., Whang, K. Y., & You, S. (2010). Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells and Development, 19, 887–902.
Zagoura, D.S., Roubelakis, M.G., Bitsika, V., Trohatou, O., Pappa, K.I., Kapelouzou, A., Antsaklis, A. & Anagnou, N.P. (2011) Therapeutic potential of a distinct population of human amniotic fluid mesenchymal stem cells and their secreted molecules in mice with acute hepatic failure. Gut
Zagoura, D. S., Trohatou, O., Bitsika, V., Makridakis, M., Pappa, K. I., Vlahou, A., Roubelakis, M. G., & Anagnou, N. P. (2013). AF-MSCs fate can be regulated by culture conditions. Cell Death and Disease, 4, e571.
Zhou, B., Tsaknakis, G., Coldwell, K. E., Khoo, C. P., Roubelakis, M. G., Chang, C. H., Pepperell, E., & Watt, S. M. (2012). A novel function for the haemopoietic supportive murine bone marrow MS-5 mesenchymal stromal cell line in promoting human vasculogenesis and angiogenesis. British Journal of Haematology, 157, 299–311.
Acknowledgments
This research was supported by Grant PENED No. 03ED 652 from the Greek Secretariat of Research and Technology and the European Union and by Grant Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework Research Funding Program: Heracleitus II, Investing in Knowledge Society through the European Social Fund. We are grateful to Professor Suzanne M. Watt (NDCLS, University of Oxford) for providing DF samples.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Authorship Contribution
M.G. Roubelakis: Conception and design, experimental procedures, data analysis, data approval and manuscript writing.
O. Trohatou: Experimental procedures, data analysis and manuscript reviewing
A. Roubelakis: PRP samples provision, patient treatment and surveillance, data analysis and manuscript reviewing.
E. Mili: PRP collection, patient treatment, experimental procedures, data analysis
I. Kalaitzopoulos: PRP samples provision, patient treatment and surveillance
Georgios Papazoglou: Patient treatment and surveillance
K.I. Pappa: Amniotic fluid samples provision
N.P. Anagnou: Financial support and manuscript reviewing
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roubelakis, M.G., Trohatou, O., Roubelakis, A. et al. Platelet-Rich Plasma (PRP) Promotes Fetal Mesenchymal Stem/Stromal Cell Migration and Wound Healing Process. Stem Cell Rev and Rep 10, 417–428 (2014). https://doi.org/10.1007/s12015-013-9494-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-013-9494-8