Abstract
Background
Bone marrow derived mesenchymal stem cells (BM-MSCs) are used extensively in transplantation but their use is associated with many problems including low abundance in BM, low overall number, decreased differentiation potential with age and the invasive isolation procedures needed to obtain BM. We report a novel method of isolating placental MSCs (pMSCs) from chorionic villi, which exhibit the phenotypic and functional characteristics that will make them an attractive source of MSCs for cell-based therapy.
Methods
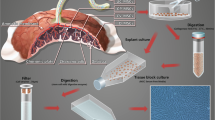
A novel explant approach was used to isolate pMSCs from chorionic villi of human placentae. These pMSCs were characterized by flow cytometry and were differentiated into adipocytes, osteocytes and chondrocytes using differentiation medium as demonstrated by cytochemical staining. The gene and protein expression profiles of pMSCs were also characterized using real time polymerase chain reaction (PCR) and flow cytometry, respectively. In addition, cytokine secretion by pMSCs was also analysed using sandwich enzyme-linked immunosorbent assay (ELISA) technique. Moreover, the migration and proliferation potentials of pMSCs were also determined.
Results
pMSCs were isolated from fetal part of the chorionic villi and these pMSCs expressed CD44, CD90, CD105, CD146, CD166 and HLA-ABC but not CD14, CD19, CD40, CD45, CD80, CD83, CD86 and HLA-DR. In addition, these pMSCs differentiated into osteocytes, chondrocytes and adipocytes and they also expressed several adhesion molecules, chemokines/receptors, growth factor receptors and cytokines/receptors. Moreover, they secreted many cytokines (IL-1Ra, IL6, IL8, IL10, IL11 and IL15) and they were able to proliferate. Furthermore, they migrated in response to chemotactic factors including stromal cell-derived factor-1 (SDF-1), platelet derived growth factor (PDGF), hepatocyte growth factor (HGF), and monocyte chemotactic protein-1 (MCP-1).
Conclusions
We devised a novel explant method of isolating pMSCs that expressed many biological factors responsible for mediating cellular processes such as migration/homing, immune modulation and angiogenesis. Therefore, we suggest that pMSCs prepared from human term placental chorionic villous explants are an attractive source of MSCs for cell therapy.





Similar content being viewed by others
References
Friedenstein, A. J., Chailakhjan, R. K., & Lalykina, K. S. (1970). The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell and Tissue Kinetics, 3, 393–403.
Augello, A., Kurth, T. B., & De Bari, C. (2010). Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. European Cells & Materials, 20, 121–133.
Krampera, M., Pizzolo, G., Aprili, G., & Franchini, M. (2006). Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone, 39, 678–683.
Phinney, D. G., & Prockop, D. J. (2007). Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells, 25, 2896–2902.
Tanaka, M., & Miyajima, A. (2012). Identification and isolation of adult liver stem/progenitor cells. Methods in Molecular Biology, 826, 25–32.
Sakaguchi, Y., Sekiya, I., Yagishita, K., & Muneta, T. (2005). Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis and Rheumatism, 52, 2521–2529.
Roubelakis, M. G., Pappa, K. I., Bitsika, V., et al. (2007). Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells and Development, 16, 931–952.
In’t Anker, P. S., Scherjon, S. A., Kleijburg-van der Keur, C., et al. (2004). Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells, 22, 1338–1345.
Kanematsu, D., Shofuda, T., Yamamoto, A., et al. (2011). Isolation and cellular properties of mesenchymal cells derived from the decidua of human term placenta. Differentiation; Research in Biological Diversity, 82, 77–88.
Pelagiadis, I., Relakis, K., Kalmanti, L., & Dimitriou, H. (2012). CD133 immunomagnetic separation: effectiveness of the method for CD133(+) isolation from umbilical cord blood. Cytotherapy.
Gronthos, S., Arthur, A., Bartold, P. M., & Shi, S. (2011). A method to isolate and culture expand human dental pulp stem cells. Methods in Molecular Biology, 698, 107–121.
Dominici, M., Le Blanc, K., Mueller, I., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8, 315–317.
Le Blanc, K., Frassoni, F., Ball, L., et al. (2008). Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet, 371, 1579–1586.
Toubai, T., Paczesny, S., Shono, Y., et al. (2009). Mesenchymal stem cells for treatment and prevention of graft-versus-host disease after allogeneic hematopoietic cell transplantation. Current Stem Cell Research & Therapy, 4, 252–259.
Majumdar, M. K., Thiede, M. A., Haynesworth, S. E., Bruder, S. P., & Gerson, S. L. (2000). Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. Journal of Hematotherapy & Stem Cell Research, 9, 841–848.
D’Ippolito, G., Schiller, P. C., Ricordi, C., Roos, B. A., & Howard, G. A. (1999). Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 14, 1115–1122.
Brooke, G., Tong, H., Levesque, J. P., & Atkinson, K. (2008). Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells and Development, 17, 929–940.
Fukuchi, Y., Nakajima, H., Sugiyama, D., Hirose, I., Kitamura, T., & Tsuji, K. (2004). Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells, 22, 649–658.
Yen, B. L., Huang, H. I., Chien, C. C., et al. (2005). Isolation of multipotent cells from human term placenta. Stem Cells, 23, 3–9.
Ringe, J., Strassburg, S., Neumann, K., et al. (2007). Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. Journal of Cellular Biochemistry, 101, 135–146.
Li, D., Wang, G. Y., Dong, B. H., Zhang, Y. C., Wang, Y. X., & Sun, B. C. (2007). Biological characteristics of human placental mesenchymal stem cells and their proliferative response to various cytokines. Cells, Tissues, Organs, 186, 169–179.
Ponte, A. L., Marais, E., Gallay, N., et al. (2007). The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells, 25, 1737–1745.
Zhang, X., Mitsuru, A., Igura, K., et al. (2006). Mesenchymal progenitor cells derived from chorionic villi of human placenta for cartilage tissue engineering. Biochemical and Biophysical Research Communications, 340, 944–952.
Igura, K., Zhang, X., Takahashi, K., Mitsuru, A., Yamaguchi, S., & Takashi, T. A. (2004). Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy, 6, 543–553.
Hirota, M., Ohmuraya, M., & Baba, H. (2006). The role of trypsin, trypsin inhibitor, and trypsin receptor in the onset and aggravation of pancreatitis. Journal of Gastroenterology, 41, 832–836.
Castrechini, N. M., Murthi, P., Gude, N. M., et al. (2010). Mesenchymal stem cells in human placental chorionic villi reside in a vascular Niche. Placenta, 31, 203–212.
Gronthos, S., Franklin, D. M., Leddy, H. A., Robey, P. G., Storms, R. W., & Gimble, J. M. (2001). Surface protein characterization of human adipose tissue-derived stromal cells. Journal of Cellular Physiology, 189, 54–63.
Ji, J. F., He, B. P., Dheen, S. T., & Tay, S. S. (2004). Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells, 22, 415–427.
Murphy, P. M., Baggiolini, M., Charo, I. F., et al. (2000). International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacological Reviews, 52, 145–176.
Kim, D. H., Yoo, K. H., Choi, K. S., et al. (2005). Gene expression profile of cytokine and growth factor during differentiation of bone marrow-derived mesenchymal stem cell. Cytokine, 31, 119–126.
Segers, V. F., Van Riet, I., Andries, L. J., et al. (2006). Mesenchymal stem cell adhesion to cardiac microvascular endothelium: activators and mechanisms. American Journal of Physiology. Heart and Circulatory Physiology, 290, H1370–H1377.
Yong, K. L., Watts, M., Shaun Thomas, N., Sullivan, A., Ings, S., & Linch, D. C. (1998). Transmigration of CD34+ cells across specialized and nonspecialized endothelium requires prior activation by growth factors and is mediated by PECAM-1 (CD31). Blood, 91, 1196–1205.
Kagimoto, Y., Yamada, H., Ishikawa, T., et al. (2008). A regulatory role of interleukin 15 in wound healing and mucosal infection in mice. Journal of Leukocyte Biology, 83, 165–172.
Abumaree, M., Al Jumah, M., Pace, R. A., & Kalionis, B. (2011). Immunosuppressive properties of mesenchymal stem cells. Stem Cell Reviews.
Perrigoue, J. G., Zaph, C., Guild, K., Du, Y., & Artis, D. (2009). IL-31-IL-31R interactions limit the magnitude of Th2 cytokine-dependent immunity and inflammation following intestinal helminth infection. Journal of Immunology, 182, 6088–6094.
Artis, D., Villarino, A., Silverman, M., et al. (2004). The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. Journal of Immunology, 173, 5626–5634.
Nold, M. F., Nold-Petry, C. A., Pott, G. B., et al. (2008). Endogenous IL-32 controls cytokine and HIV-1 production. Journal of Immunology, 181, 557–565.
Andoh, A., Shioya, M., Nishida, A., et al. (2009). Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. Journal of Immunology, 183, 687–695.
Su, Z., Emdad, L., Sauane, M., et al. (2005). Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene, 24, 7552–7566.
Suh, W. K., Gajewska, B. U., Okada, H., et al. (2003). The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nature Immunology, 4, 899–906.
Starke, A., Lindenmeyer, M. T., Segerer, S., et al. (2010). Renal tubular PD-L1 (CD274) suppresses alloreactive human T-cell responses. Kidney International, 78, 38–47.
Singh, A. K., Stock, P., & Akbari, O. (2011). Role of PD-L1 and PD-L2 in allergic diseases and asthma. Allergy, 66, 155–162.
Jones, B. J., Brooke, G., Atkinson, K., & McTaggart, S. J. (2007). Immunosuppression by placental indoleamine 2,3-dioxygenase: a role for mesenchymal stem cells. Placenta, 28, 1174–1181.
Acknowledgments
We would also like to thank the staff and patients of the Delivery Unit, King Abdul Aziz Medical City for their help in obtaining placentae. This study was supported by grants from King Abdulla International Medical Research Centre (Grant No. RC08/114) and King Abdulaziz City for Science and Technology (Grant No. ARP-29-186). B. Kalionis acknowledges that support of National Health and Medical Research Grant 509178.
Conflict of interest The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abumaree, M.H., Al Jumah, M.A., Kalionis, B. et al. Phenotypic and Functional Characterization of Mesenchymal Stem Cells from Chorionic Villi of Human Term Placenta. Stem Cell Rev and Rep 9, 16–31 (2013). https://doi.org/10.1007/s12015-012-9385-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-012-9385-4