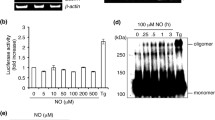
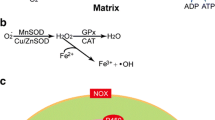
Abstract
Elevated levels of mitochondrial nitrosative stress have been associated with the pathogenesis of both Parkinson’s and Alzheimer’s diseases. The mechanism involves catalytic poisoning of the endoplasmic reticulum (ER)—resident oxidoreductase chaperone, protein disulfide isomerase (PDI), and the subsequent accumulation of ER-processed substrate proteins. Using a model system to mimic mitochondrial oxidative and nitrosative stress, we demonstrate a PDI-independent mechanism whereby reactive oxygen species (ROS) compromise regeneration rates of disulfide bond-containing ER-processed proteins. Under ROS-duress, the secretion-destined traffic adopts disulfide-exposed structures making the protein flux retrotranslocation biased. We also demonstrate that ROS-compromised protein maturation rates can be rescued by the polyphenol ellagic acid (EA). Our results are significant in that they reveal an additional mechanism which could promote neurodegenerative disorders. Furthermore, our data reveal that EA possesses therapeutic potential as a lead prophylactic agent against oxidative/nitrosative stress-related neurodegenerative diseases.



Similar content being viewed by others
References
Narayan, M., Welker, E., Wedemeyer, W. J., & Scheraga, H. A. (2000). Oxidative folding of proteins. Accounts of Chemical Research, 33, 737–820.
Woycechowsky, K. J., & Raines, R. T. (2000). Native disulfide bond formation in proteins. Current Opinion in Chemical Biology, 4, 533–539.
Arolas, J. L., Aviles, F. X., Chang, J. Y., & Ventura, S. (2006). Folding of small disulfide-rich proteins: clarifying the puzzle. Trends in Biochemical Sciences, 31, 292–301.
Wedemeyer, W. J., Welker, E., Narayan, M., & Scheraga, H. A. (2000). Disulfide bonds and protein folding. Biochemistry, 39, 4207–4216.
Welker, E., Wedemeyer, W. J., Narayan, M., & Scheraga, H. A. (2001). Coupling of conformational folding and disulfide-bond reactions in oxidative folding of proteins. Biochemistry, 40, 9059–9064.
Welker, E., Narayan, M., Wedemeyer, W. J., & Scheraga, H. A. (2001). Structural determinants of oxidative folding in proteins. Proceedings of the National Academy of Sciences, 98, 2312–2316.
Tu, B. P., & Weissman, J. S. (2004). Oxidative protein folding in eukaryotes: mechanisms and consequences. Journal of Cell Biology, 164, 341–346.
Wilkinson, B., & Gilbert, H. F. (2004). Protein disulfide isomerase. Biochimica et Biophysica Acta, 1699, 35–44.
Tian, G., Xiang, S., Noiva, R., Lennarz, W. J., & Schindelin, H. (2006). The crystal structure of yeast protein disulfide isomerase suggests cooperativity between its active sites. Cell, 124, 61–73. Erratum in: Cell, 124 (2006) 1085–1088.
Gilbert, H. F. (1998). Protein disulfide isomerase. Methods in Enzymology, 290, 26–50.
Uehara, T., Nakamura, T., Yao, D., Shi, Z. Q., Gu, Z., Ma, Y., et al. (2006). S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature, 441, 513–517.
Kabiraj, P., Marin, E. J., Varela-Ramirez, A., Zubia, E., & Narayan, M. (2014). Ellagic acid mitigates SNO-PDI induced aggregation of parkinsonian biomarkers. ACS Chemical Neuroscience, 5, 1209–1220.
Kabiraj, P., Pal, R., Varela-Ramirez, A., Miranda, M., & Narayan, M. (2012). Nitrosative stress mediated misfolded protein aggregation mitigated by Na-d-β-hydroxybutyrate intervention. Biochemical and Biophysical Research Communications, 426, 438–444.
Pal, R., Miranda, M., & Narayan, M. (2011). Nitrosative stress-induced Parkinsonian Lewy-like aggregates prevented through polyphenolic phytochemical analog intervention. Biochemical and Biophysical Research Communications, 404, 324–329.
Benham, A. M. (2012). The protein disulfide isomerase (pdi) family: key players in health and disease. Antioxidants & Redox Signaling, 16, 781–789.
Benhar, M., Forrester, M. T., & Stamler, J. S. (2006). Nitrosative stress in the ER: A new role for S-nitrosylation in neurodegenerative diseases. ACS Chemical Biology, 1, 355–358.
Nakamura, T., Prikhodko, O. A., Pirie, E., Nagar, S., Akhtar, M. W., Oh, C. K., et al. (2015). Aberrant protein S-nitrosylation contributes to the pathophysiology of neurodegenerative diseases. Neurobiology of Disease,. doi:10.1016/j.nbd.2015.03.017.
Jeon, G. S., Nakamura, T., Lee, J. S., Choi, W. J., Ahn, S. W., Lee, K. W., et al. (2014). Potential effect of S-nitrosylated protein disulfide isomerase on mutant SOD1 aggregation and neuronal cell death in amyotrophic lateral sclerosis. Molecular Neurobiology, 49, 796–807.
Gu, Z., Nakamura, T., & Lipton, S. A. (2010). Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Molecular Neurobiology, 41, 55–72.
Conway, M. E., & Harris, M. (2015). S-nitrosylation of the thioredoxin-like domains of protein disulfide isomerase and its role in neurodegenerative conditions. Frontiers in Chemistry, 3, 27.
Nakamura, T., Prikhodko, O. A., Pirie, E., Nagar, S., Akhtar, M. W., Oh, C. K., et al. (2015). Aberrant protein S-nitrosylation contributes to the pathophysiology of neurodegenerative diseases. Neurobiology of Disease,. doi:10.1016/j.nbd.2015.03.017.
Chaudhari, N., Talwar, P., Parimisetty, A., Lefebvre d’Hellencourt, C., & Ravanan, P. (2014). A molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neuroscience, 8, 213.
Wang, Y. H., & Narayan, M. (2008). pH dependence of the isomerase activity of protein disulfide isomerase: Insights into its functional relevance. Protein Journal, 27, 181–185.
Pal, R., Cristan, E. A., Schnittker, K., & Narayan, M. (2010). Rescue of ER oxidoreductase function through polyphenolic phytochemical intervention: implications for subcellular traffic and neurodegenerative disorders. Biochemical and Biophysical Research Communications, 392, 567–571.
Fink, M., Nieves, P., Chang, S., & Narayan, M. (2008). Non-redox-active small-molecules can accelerate oxidative protein folding by novel mechanisms. Biophysical Chemistry, 132, 104–109.
Hseu, Y. C., Chou, C. W., Senthil Kumar, K. J., Fu, K. T., Wang, M. H., Hsu, L. S., et al. (2012). Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food and Chemical Toxicology, 50, 1245–1255.
Kilic, I., Yeşiloğlu, Y., & Bayrak, Y. (2014). Spectroscopic studies on the antioxidant activity of ellagic acid. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 130, 447–452. doi:10.1016/j.saa.2014.04.052.
Sirimulla, S., Bailey, J. B., Vegesna, R., & Narayan, M. (2013). Halogen interactions in protein-ligand complexes: implications of halogen bonding for rational drug design. Journal of Chemical Information and Modeling, 53, 2781–2791.
Sirimulla, S., Pal, R., Raparla, M., Bailey, J. B., Duran, R., Altamirano, A. M., et al. (2012). Identification of novel nitrosative stress inhibitors through virtual screening and experimental evaluation. Molecular Informatics, 31, 167–172.
Rothwarf, D. M., & Scheraga, H. A. (1993). Regeneration of bovine pancreatic ribonuclease A. 1. Steady-state distribution. Biochemistry, 32(10), 2671–2679.
Rothwarf, D. M., Li, Y., & Scheraga, H. A. (1998). Regeneration of bovine pancreatic ribonuclease A: Identification of two nativelike three-disulfide intermediates involved in separate pathways. Biochemistry, 37(11), 3760–3766.
Wang, Y.-H., & Narayan, M. (2008). pH dependence of the isomerase activity of protein disulfide isomerase: insights into its functional relevance. Protein Journal, 27(3), 181–185.
Pal, R., Cristan, E. A., Schnittker, K., & Narayan, M. (2010). Rescue of ER oxidoreductase function through polyphenolic phytochemical intervention: implications for subcellular traffic and neurodegenerative disorders. Biochemical and Biophysical Research Communications, 392(4), 567–571.
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell, 149, 1060–1072.
Skouta, R., Dixon, S. J., Wang, J., et al. (2014). Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. Journal of the American Chemical Society, 136, 4551–4556.
Blois, M. S. (1958). Antioxidant determinations by the use of a stable free radical. Nature, 181, 1199–1200.
Xu, G., Narayan, M., & Scheraga, H. A. (2005). The oxidative folding rate of bovine pancreatic ribonuclease is enhanced by a covalently attached oligosaccharide. Biochemistry, 44(28), 9817–9823.
Mattson, M. P. (2006). Nitro-PDI incites toxic waste accumulation. Nature Neuroscience, 9(7), 865–867.
Uehara, T., Nakamura, T., Yao, D., Shi, Z. Q., Gu, Z., Ma, Y., et al. (2006). S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature, 441(7092), 513–517.
Acknowledgments
The authors would like to express special thanks to the staff of the Cytometry, Screening, and Imaging Core Facility of the Border Biomedical Research Center at The University of Texas at El Paso (UTEP). This facility is supported by Grant # 2G12MD007592 and Grant # 5G12MD007592 from the Research Centers in Minority Institutions program of the National Institutes on Minority Health and Health Disparities. In addition, M.N. would like to thank Dr. Eddie Vazquez and Mrs. Holly Vazquez (The El Paso Pain Center) for their financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Carlos Valenzuela and Daniella Sisniega have been contributed equally to this work.
Rights and permissions
About this article
Cite this article
Khalil, M.F., Valenzuela, C., Sisniega, D. et al. ER Protein Processing Under Oxidative Stress: Implications and Prevention. Cell Biochem Biophys 74, 213–220 (2016). https://doi.org/10.1007/s12013-016-0726-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-016-0726-9