Abstract
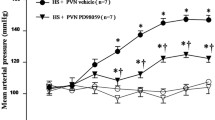
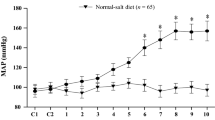
High-salt-induced inflammation and oxidative stress in the hypothalamic paraventricular nucleus (PVN) contribute to the pathogenesis of salt-sensitive hypertension. In this study, we hypothesized that chronic inhibition of nuclear factor-κB (NF-κB) activity in the PVN delays the progression of hypertension by upregulating anti-inflammatory cytokines, reducing NLRP3 (NOD-like receptor family pyrin domain containing 3) and IL-1β and attenuating p-IKKβ, NF-κB p65 activity and NAD(P)H oxidase in the PVN of salt-sensitive hypertensive rats. Dahl salt-sensitive rats received a high-salt diet (HS, 8 % NaCl) or a normal-salt diet (NS, 0.3 % NaCl) for 6 weeks and were treated with bilateral PVN infusion with either vehicle or pyrrolidine dithiocarbamate (PDTC, 5 μg/h), a NF-κB inhibitor via osmotic minipump. The mean arterial pressure and plasma levels of norepinephrine (NE) and epinephrine (EPI) were significantly increased in high-salt-fed rats. In addition, rats with high-salt diet had higher levels of p-IKKβ, NF-κB p65 activity, Fra-like (Fra-LI) activity (an indicator of chronic neuronal activation), NOX-4 (subunits of NAD(P)H oxidase), NLRP3 and IL-1β, and lower levels of IL-10 in the PVN than normal diet rats. Bilateral PVN infusions of PDTC attenuated these high-salt-induced changes. These findings suggest that high-salt-induced NF-κB activation in the PVN caused hypertension via sympathoexcitation, which are associated with the increases of NLRP3, IL-1β and oxidative stress in the PVN; PVN inhibition of NF-κB activity attenuates NLRP3, IL-1β and oxidative stress in the PVN and thereby attenuates hypertension.









Similar content being viewed by others
References
Li, N., Luo, W., Juhong, Z., Yang, J., Wang, H., Zhou, L., & Chang, J. (2010). Associations between genetic variations in the FURIN gene and hypertension. BMC Medical Genetics, 11, 124.
Dornas, W. C., & Silva, M. E. (2011). Animal models for the study of arterial hypertension. Journal of Biosciences, 36, 731–737.
Zhang, M., Qin, D. N., Suo, Y. P., Su, Q., Li, H. B., Miao, Y. W., et al. (2015). Endogenous hydrogen peroxide in the hypothalamic paraventricular nucleus regulates neurohormonal excitation in high salt-induced hypertension. Toxicology Letters, 235, 206–215.
Huang, B. S., Zheng, H., Tan, J., Patel, K. P., & Leenen, F. H. (2011). Regulation of hypothalamic renin-angiotensin system and oxidative stress by aldosterone. Experimental Physiology, 96, 1028–1038.
Qi, J., Zhang, D. M., Suo, Y. P., Song, X. A., Yu, X. J., Elks, C., et al. (2013). Renin-angiotensin system modulates neurotransmitters in the paraventricular nucleus and contributes to angiotensin II-induced hypertensive response. Cardiovascular Toxicology, 13, 48–54.
Li, H. B., Qin, D. N., Ma, L., Miao, Y. W., Zhang, D. M., Lu, Y., et al. (2014). Chronic infusion of lisinopril into hypothalamic paraventricular nucleus modulates cytokines and attenuates oxidative stress in rostral ventrolateral medulla in hypertension. Toxicology and Applied Pharmacology, 279, 141–149.
Kang, Y. M., Ma, Y., Zheng, J. P., Elks, C., Sriramula, S., Yang, Z. M., & Francis, J. (2009). Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovascular Research, 82, 503–512.
Dinarello, C. A. (2009). Immunological and inflammatory functions of the interleukin-1 family. Annual Review of Immunology, 27, 519–550.
Mohamed, I. N., Hafez, S. S., Fairaq, A., Ergul, A., Imig, J. D., & El-Remessy, A. B. (2014). Thioredoxin-interacting protein is required for endothelial NLRP3 inflammasome activation and cell death in a rat model of high-fat diet. Diabetologia, 57, 413–423.
Yu, X. J., Zhang, D. M., Jia, L. L., Qi, J., Song, X. A., Tan, H., et al. (2015). Inhibition of NF-kappaB activity in the hypothalamic paraventricular nucleus attenuates hypertension and cardiac hypertrophy by modulating cytokines and attenuating oxidative stress. Toxicology and Applied Pharmacology, 284, 315–322.
Bauernfeind, F. G., Horvath, G., Stutz, A., Alnemri, E. S., MacDonald, K., Speert, D., et al. (2009). Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. Journal of Immunology, 183, 787–791.
Cao, Y., Mu, J. J., Fang, Y., Yuan, Z. Y., & Liu, F. Q. (2013). Impact of high salt independent of blood pressure on PRMT/ADMA/DDAH pathway in the aorta of dahl salt-sensitive rats. International Journal of Molecular Sciences, 14, 8062–8072.
Kang, Y. M., Ma, Y., Elks, C., Zheng, J. P., Yang, Z. M., & Francis, J. (2008). Cross-talk between cytokines and renin-angiotensin in hypothalamic paraventricular nucleus in heart failure: Role of nuclear factor-kappaB. Cardiovascular Research, 79, 671–678.
Yu, X. J., Suo, Y. P., Qi, J., Yang, Q., Li, H. H., Zhang, D. M., et al. (2013). Interaction between AT1 receptor and NF-kappaB in hypothalamic paraventricular nucleus contributes to oxidative stress and sympathoexcitation by modulating neurotransmitters in heart failure. Cardiovascular Toxicology, 13, 381–390.
Wei, S. G., Yu, Y., Zhang, Z. H., Weiss, R. M., & Felder, R. B. (2008). Mitogen-activated protein kinases mediate upregulation of hypothalamic angiotensin II type 1 receptors in heart failure rats. Hypertension, 52, 679–686.
Okada, S., Yamaguchi-Shima, N., Shimizu, T., Arai, J., Yorimitsu, M., & Yokotani, K. (2008). Brain nuclear factor kappa B is involved in the corticotropin-releasing factor-induced central activation of sympatho-adrenomedullary outflow in rats. European Journal of Pharmacology, 584, 207–212.
Kang, Y. M., Gao, F., Li, H. H., Cardinale, J. P., Elks, C., Zang, W. J., et al. (2011). NF-kappaB in the paraventricular nucleus modulates neurotransmitters and contributes to sympathoexcitation in heart failure. Basic Research in Cardiology, 106, 1087–1097.
Francis, J., MohanKumar, S. M., & MohanKumar, P. S. (2000). Correlations of norepinephrine release in the paraventricular nucleus with plasma corticosterone and leptin after systemic lipopolysaccharide: Blockade by soluble IL-1 receptor. Brain Research, 867, 180–187.
Yi, Q. Y., Qi, J., Yu, X. J., Li, H. B., Zhang, Y., Su, Q., et al. (2015). Paraventricular nucleus infusion of epigallocatechin-3-O-gallate improves renovascular hypertension. Cardiovascular Toxicology. doi:10.1007/s12012-015-9335-x.
Kang, Y. M., Zhang, A. Q., Zhao, X. F., Cardinale, J. P., Elks, C., Cao, X. M., et al. (2011). Paraventricular nucleus corticotrophin releasing hormone contributes to sympathoexcitation via interaction with neurotransmitters in heart failure. Basic Research in Cardiology, 106, 473–483.
Chen, A. D., Zhang, S. J., Yuan, N., Xu, Y., De, W., Gao, X. Y., & Zhu, G. Q. (2011). Angiotensin AT1 receptors in paraventricular nucleus contribute to sympathetic activation and enhanced cardiac sympathetic afferent reflex in renovascular hypertensive rats. Experimental Physiology, 96, 94–103.
Zhu, G. Q., Gao, L., Patel, K. P., Zucker, I. H., & Wang, W. (2004). ANG II in the paraventricular nucleus potentiates the cardiac sympathetic afferent reflex in rats with heart failure. Journal of Applied Physiology, 97, 1746–1754.
Li, H. B., Qin, D. N., Cheng, K., Su, Q., Miao, Y. W., Guo, J., et al. (2015). Central blockade of salusin beta attenuates hypertension and hypothalamic inflammation in spontaneously hypertensive rats. Scientific Reports, 5, 11162.
Su, Q., Qin, D. N., Wang, F. X., Ren, J., Li, H. B., Zhang, M., et al. (2014). Inhibition of reactive oxygen species in hypothalamic paraventricular nucleus attenuates the renin-angiotensin system and proinflammatory cytokines in hypertension. Toxicology and Applied Pharmacology, 276, 115–120.
Kang, Y. M., He, R. L., Yang, L. M., Qin, D. N., Guggilam, A., Elks, C., et al. (2009). Brain tumour necrosis factor-alpha modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovascular Research, 83, 737–746.
MohanKumar, S. M., MohanKumar, P. S., & Quadri, S. K. (1998). Specificity of interleukin-1beta-induced changes in monoamine concentrations in hypothalamic nuclei: Blockade by interleukin-1 receptor antagonist. Brain Research Bulletin, 47, 29–34.
Kang, Y. M., Zhang, Z. H., Johnson, R. F., Yu, Y., Beltz, T., Johnson, A. K., et al. (2006). Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circulation Research, 99, 758–766.
Sriramula, S., Cardinale, J. P., Lazartigues, E., & Francis, J. (2011). ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovascular Research, 92, 401–408.
Miller, F. J, Jr, Gutterman, D. D., Rios, C. D., Heistad, D. D., & Davidson, B. L. (1998). Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circulation Research, 82, 1298–1305.
Li, Z. W., Chu, W., Hu, Y., Delhase, M., Deerinck, T., Ellisman, M., et al. (1999). The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. The Journal of Experimental Medicine, 189, 1839–1845.
Wei, J., & Liang, B. S. (2012). PPM1B and P-IKKbeta expression levels correlated inversely with rat gastrocnemius atrophy after denervation. Brazilian Journal of Medical and Biological Research, 45, 711–715.
Sandanger, O., Ranheim, T., Vinge, L. E., Bliksoen, M., Alfsnes, K., Finsen, A. V., et al. (2013). The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovascular Research, 99, 164–174.
Tschopp, J., Martinon, F., & Burns, K. (2003). NALPs: A novel protein family involved in inflammation. Nature Reviews Molecular Cell Biology, 4, 95–104.
Bronner, D. N., Abuaita, B. H., Chen, X., Fitzgerald, K. A., Nunez, G., He, Y., et al. (2015). Endoplasmic reticulum stress activates the inflammasome via NLRP3-and caspase-2-driven mitochondrial damage. Immunity, 43, 451–462.
Kim, S., Joe, Y., Jeong, S. O., Zheng, M., Back, S. H., Park, S. W., et al. (2014). Endoplasmic reticulum stress is sufficient for the induction of IL-1beta production via activation of the NF-kappaB and inflammasome pathways. Innate immunity, 20, 799–815.
Acknowledgments
This study was supported by National Basic Research Program of China (No. 2012CB517805) and National Natural Science Foundation of China (Nos. 91439120, 81370356, 81170248, 81471471). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Qi, J., Yu, XJ., Shi, XL. et al. NF-κB Blockade in Hypothalamic Paraventricular Nucleus Inhibits High-Salt-Induced Hypertension Through NLRP3 and Caspase-1. Cardiovasc Toxicol 16, 345–354 (2016). https://doi.org/10.1007/s12012-015-9344-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-015-9344-9