Abstract
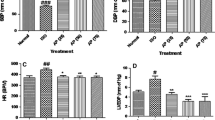
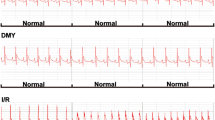
Apigenin (Api), a mainly bioactive component of Apium graveolens L. var. dulce DC. (a traditional Chinese medicinal herb), possesses a wide range of biological activities, including antioxidant effects. It also has been shown to associate with lower prevalence of cardiovascular diseases, but its mechanisms of action remain unclear. The aim of the present study is to investigate the role of Api in isolated rat heart model of ischemia/reperfusion (I/R). Langendorff-perfused isolated rat hearts were used in our study. Api was added to the perfusate before ischemia and during reperfusion in the isolated pulsed rat heart exposed to 30-min ischemia followed by 50-min reperfusion. The treatment with Api conferred a cardioprotective effect, and the treated hearts demonstrated an improved ischemic cardiac functional recovery, a decreased myocardial infarct size, a reduced activities of creatine kinase isoenzyme and lactate dehydrogenase in the coronary flow, a reduced number of apoptotic cardiomyocytes, a reduced activity of caspase-3, up-regulation of the anti-apoptotic protein Bcl-2 and down-regulation of the pro-apoptotic protein Bax. In addition, Api inhibited the phosphorylation of p38 MAPKS during I/R. In conclusion, these observations provide preliminary evidence that Api can protect cardiomyocytes from I-/R-induced injury, at least partially, through the inhibition of p38 MAPKS signaling pathway.









Similar content being viewed by others
Abbreviations
- CK-MB:
-
Creatine kinase-MB isoform
- I/R:
-
Ischemia–reperfusion
- K–H:
-
Krebs–Henseleit
- LDH:
-
Lactate dehydrogenase
- TTC:
-
2,3,5-Triphenyltetrazolium chloride
- LVEDP:
-
Left ventricular end-diastolic pressure
References
Sharma, H., Kanwal, R., Bhaskaran, N., & Gupta, S. (2014). Plant flavone apigenin binds to nucleic acid bases and reduces oxidative DNA damage in prostate epithelial cells. PLoS ONE, 9, e91588.
Lu, X. Y., Sun, D. L., Chen, Z. J., Chen, T., Li, L. P., Xu, Z. H., et al. (2010). Relative contribution of small and large intestine to deglycosylation and absorption of flavonoids from Chrysanthemum morifolium extract. Journal of Agriculture and Food Chemistry, 58, 10661–10667.
Beara, I. N., Lesjak, M. M., Jovin, E. D., Balog, K. J., Anackov, G. T., Orcic, D. Z., et al. (2009). Plantain (Plantago L.) species as novel sources of flavonoid antioxidants. Journal of Agriculture and Food Chemistry, 57, 9268–9273.
Kanazawa, K., Uehara, M., Yanagitani, H., & Hashimoto, T. (2006). Bioavailable flavonoids to suppress the formation of 8-OHdG in HepG2 cells. Archives of Biochemistry and Biophysics, 455, 197–203.
Zhang, Y. H., Park, Y. S., Kim, T. J., Fang, L. H., Ahn, H. Y., Hong, J. T., et al. (2000). Endothelium-dependent vasorelaxant and antiproliferative effects of apigenin. General Pharmacology, 35, 341–347.
Basile, A., Giordano, S., Lopez-Saez, J. A., & Cobianchi, R. C. (1999). Antibacterial activity of pure flavonoids isolated from mosses. Phytochemistry, 52, 1479–1482.
Gupta, S., Afaq, F., & Mukhtar, H. (2002). Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene, 21, 3727–3738.
Lindenmeyer, F., Li, H., Menashi, S., Soria, C., & Lu, H. (2001). Apigenin acts on the tumor cell invasion process and regulates protease production. Nutrition and Cancer, 39, 139–147.
Jin, B. H., Qian, L. B., Chen, S., Li, J., Wang, H. P., Bruce, I. C., et al. (2009). Apigenin protects endothelium-dependent relaxation of rat aorta against oxidative stress. European Journal of Pharmacology, 616, 200–205.
Meyer, H., Bolarinwa, A., Wolfram, G., & Linseisen, J. (2006). Bioavailability of apigenin from apiin-rich parsley in humans. Annals of Nutrition and Metabolism, 50, 167–172.
Bellosta, S., Bogani, P., Canavesi, M., Galli, C., & Visioli, F. (2008). Mediterranean diet and cardioprotection: Wild artichoke inhibits metalloproteinase 9. Molecular Nutrition and Food Research, 52, 1147–1152.
Jeong, C. W., Yoo, K. Y., Lee, S. H., Jeong, H. J., Lee, C. S., & Kim, S. J. (2012). Curcumin protects against regional myocardial ischemia/reperfusion injury through activation of RISK/GSK-3beta and inhibition of p38 MAPK and JNK. Journal of Cardiovascular Pharmacology and Therapeutics, 17, 387–394.
Yang, Y., Hu, S. J., Li, L., & Chen, G. P. (2009). Cardioprotection by polysaccharide sulfate against ischemia/reperfusion injury in isolated rat hearts. Acta Pharmacologica Sinica, 30, 54–60.
Schwertz, H., Carter, J. M., Abdudureheman, M., Russ, M., Buerke, U., Schlitt, A., et al. (2007). Myocardial ischemia/reperfusion causes VDAC phosphorylation which is reduced by cardioprotection with a p38 MAP kinase inhibitor. Proteomics, 7, 4579–4588.
Lin, M., Lu, S. S., Wang, A. X., Qi, X. Y., Zhao, D., Wang, Z. H., et al. (2011). Apigenin attenuates dopamine-induced apoptosis in melanocytes via oxidative stress-related p38, c-Jun NH2-terminal kinase and Akt signaling. Journal of Dermatological Science, 63, 10–16.
Noh, H. J., Sung, E. G., Kim, J. Y., Lee, T. J., & Song, I. H. (2010). Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by apigenin via the inhibition of p38 mitogen-activated protein kinase-dependent matrix metalloproteinase-9 expression. Oncology Reports, 24, 277–283.
Huang, C. H., Kuo, P. L., Hsu, Y. L., Chang, T. T., Tseng, H. I., Chu, Y. T., et al. (2010). The natural flavonoid apigenin suppresses Th1- and Th2-related chemokine production by human monocyte THP-1 cells through mitogen-activated protein kinase pathways. Journal of Medicinal Food, 13, 391–398.
Olfert, E. D., Cross, B. M., & McWilliam, A. A. (1993). Guide to the care and use of experimental animals. Vol. 1. No. 2. Ottawa: Canadian Council on Animal Care.
Zheng, Z., Yang, M., Zhang, F., Yu, J., Wang, J., Ma, L., et al. (2011). Gender-related difference of sevoflurane postconditioning in isolated rat hearts: Focus on phosphatidylinositol-3-kinase/Akt signaling. Journal of Surgical Research, 170, e3–e9.
Miao, Q., Wang, S., Miao, S., Wang, J., Xie, Y., & Yang, Q. (2011). Cardioprotective effect of polydatin against ischemia/reperfusion injury: Roles of protein kinase C and mito K(ATP) activation. Phytomedicine, 19, 8–12.
Omura, T., Yoshiyama, M., Ishikura, F., Kobayashi, H., Takeuchi, K., Beppu, S., et al. (2001). Myocardial ischemia activates the JAK-STAT pathway through angiotensin II signaling in in vivo myocardium of rats. Journal of Molecular and Cellular Cardiology, 33, 307–316.
Hu, J., Wang, Z., Guo, Y. Y., Zhang, X. N., Xu, Z. H., Liu, S. B., et al. (2009). A role of periaqueductal grey NR2B-containing NMDA receptor in mediating persistent inflammatory pain. Molecular Pain, 5, 71.
Pagliaro, P., Mancardi, D., Rastaldo, R., Penna, C., Gattullo, D., Miranda, K. M., et al. (2003). Nitroxyl affords thiol-sensitive myocardial protective effects akin to early preconditioning. Free Radical Biology and Medicine, 34, 33–43.
Cory, S., & Adams, J. M. (2002). The Bcl2 family: Regulators of the cellular life-or-death switch. Nature Reviews Cancer, 2, 647–656.
Antonsson, B. (2004). Mitochondria and the Bcl-2 family proteins in apoptosis signaling pathways. Molecular and Cellular Biochemistry, 256–257, 141–155.
Kuwana, T., Mackey, M. R., Perkins, G., Ellisman, M. H., Latterich, M., Schneiter, R., et al. (2002). Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell, 111, 331–342.
Burlacu, A. (2003). Regulation of apoptosis by Bcl-2 family proteins. Journal of Cellular and Molecular Medicine, 7, 249–257.
Ling, Q., Xu, X., Wei, X., Wang, W., Zhou, B., Wang, B., et al. (2011). Oxymatrine induces human pancreatic cancer PANC-1 cells apoptosis via regulating expression of Bcl-2 and IAP families, and releasing of cytochrome c. Journal of Experimental and Clinical Cancer Research, 30, 66.
Yue, T. L., Wang, C., Gu, J. L., Ma, X. L., Kumar, S., Lee, J. C., et al. (2000). Inhibition of extracellular signal-regulated kinase enhances ischemia/reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circulation Research, 86, 692–699.
Antonsson, B. (2001). Bax and other pro-apoptotic Bcl-2 family “killer-proteins” and their victim the mitochondrion. Cell and Tissue Research, 306, 347–361.
Alnemri, E. S., Livingston, D. J., Nicholson, D. W., Salvesen, G., Thornberry, N. A., Wong, W. W., et al. (1996). Human ICE/CED-3 protease nomenclature. Cell, 87, 171.
Thomas, C. J., Ng, D. C., Patsikatheodorou, N., Limengka, Y., Lee, M. W., Darby, I. A., et al. (2011). Cardioprotection from ischaemia-reperfusion injury by a novel flavonol that reduces activation of p38 MAPK. European Journal of Pharmacology, 658, 160–167.
Kaiser, R. A., Bueno, O. F., Lips, D. J., Doevendans, P. A., Jones, F., Kimball, T. F., et al. (2004). Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. Journal of Biological Chemistry, 279, 15524–15530.
Li, G., Barrett, E. J., Barrett, M. O., Cao, W., & Liu, Z. (2007). Tumor necrosis factor-alpha induces insulin resistance in endothelial cells via a p38 mitogen-activated protein kinase-dependent pathway. Endocrinology, 148, 3356–3363.
Bassi, R., Heads, R., Marber, M. S., & Clark, J. E. (2008). Targeting p38-MAPK in the ischaemic heart: Kill or cure? Current Opinion in Pharmacology, 8, 141–146.
Ma, L., Liu, H., Xie, Z., Yang, S., Xu, W., Hou, J., et al. (2014). Ginsenoside Rb3 protects cardiomyocytes against ischemia–reperfusion injury via the inhibition of JNK-mediated NF-κB pathway: A mouse cardiomyocyte model. PLoS ONE, 9, e103628.
Nicholas, R. L., Colleen, J. T., Lokugan, S. S., Yvonne, Y. Y., Suwan, Y., James, R. B., et al. (2013). Cardioprotective 3prime, 4prime-dihydroxyflavonol attenuation of JNK and p38MAPK signalling involves CaMKII inhibition. Biochemical Journal, 456, 149–161.
Jones, W. K., Brown, M., Ren, X., He, S., & McGuinness, M. (2003). NF-κB as an integrator of diverse signaling pathways. Cardiovascular Toxicology, 3, 229–253.
Chen, H.-M., Hsu, J.-H., Liou, S.-F., Chen, T.-J., Chen, L.-Y., Chiu, C.-C., et al. (2014). Baicalein, an active component of Scutellaria baicalensis Georgi, prevents lysophosphatidylcholine-induced cardiac injury by reducing reactive oxygen species production, calcium overload and apoptosis via MAPK pathways. BMC complementary and alternative medicine, 14, 233.
Acknowledgments
We thank Xiao Cao Botanical Development Co., Ltd (Xi’an, China) for supplying us with the standardized Api extract used in this study. We are grateful for the financial support by National Natural Science Foundation of China (81400276).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jing Hu, Zi-lin Li and Li-ting Xu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hu, J., Li, Z., Xu, Lt. et al. Protective Effect of Apigenin on Ischemia/Reperfusion Injury of the Isolated Rat Heart. Cardiovasc Toxicol 15, 241–249 (2015). https://doi.org/10.1007/s12012-014-9290-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-014-9290-y