Abstract
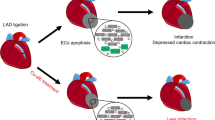
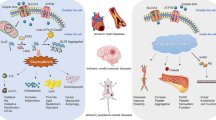
Myocardial ischemia is a primary cause for the loss of vital components such as cardiomyocytes in the heart, leading to myocardial infarction and eventual cardiac dysfunction or heart failure. Suppressed angiogenesis plays a determinant role in the pathogenesis of myocardial infarction. In response to myocardial ischemia, hypoxia-inducible factor-1α and 2α (HIF-1α and HIF-2α) accumulate in cardiomyocytes and other cell types. This would up-regulate the expression of genes involved in angiogenesis such as vascular endothelial growth factor (VEGF); however, it is often observed that the angiogenic capacity is suppressed rather than enhanced. Ischemic toxicity, which has not been fully recognized, is highly responsible for the compromised angiogenic capacity. One of the toxic effects resulting from myocardial ischemia is the loss of copper content in the heart. Although the reason for this loss has not been elucidated, the essential role of copper in the regulation of HIF-1 transcriptional activity has been described. Copper does not affect the accumulation of HIF-1α in the cell, but is required for the HIF-1 transcriptional complex formation and its interaction with the hypoxia-responsive element in target genes. Copper supplementation can stimulate the transcriptional activity of HIF-1 and restore angiogenic capacity, leading to increased capillary density in the heart. The recognition of ischemic toxicity and the effort to overcome the toxic effect would help develop alternative approaches in the treatment of ischemic heart disease.


Similar content being viewed by others
Abbreviations
- ARNT:
-
Aryl hydrocarbon nuclear translocator
- CCS:
-
Copper chaperon for Cu, Zn-superoxide dismutase
- CFF:
-
Coronary flow fraction
- FIH-1:
-
Factor inhibiting HIF-1 (hypoxia-inducible factor-1)
- Glut-1:
-
Glucose transport-1
- HER2:
-
Human epidermal growth factor receptor 2
- HIF-1α:
-
Hypoxia-inducible factor-1α
- HIF-2α:
-
Hypoxia-inducible factor-2α
- HO-1:
-
Heme oxygenase-1
- HRE:
-
Hypoxia-responsive element
- IGF:
-
Insulin-like growth factor
- iNOS:
-
Inducible nitric oxide synthase
- PHDs:
-
Prolyl hydroxylase domain–containing proteins
- PI3-kinases:
-
Phosphatidylinositol 3-kinases
- pVHL:
-
Von Hippel–Lindau protein
- TEPA:
-
Tetraethylenepentamine
- VEGF:
-
Vascular endothelial growth factor
References
Goswami, S. K., & Das, D. K. (2010). Oxygen sensing, cardiac ischemia, HIF-1alpha and some emerging concepts. Current Cardiology Reviews, 6, 265–273.
Tekin, D., Dursun, A. D., & Xi, L. (2010). Hypoxia inducible factor 1 (HIF-1) and cardioprotection. Acta Pharmacologica Sinica, 31, 1085–1094.
Eckle, T., Kohler, D., Lehmann, R., El Kasmi, K., & Eltzschig, H. K. (2008). Hypoxia-inducible factor-1 is central to cardioprotection: A new paradigm for ischemic preconditioning. Circulation, 118, 166–175.
Bautista, L., Castro, M. J., López-Barneo, J., & Castellano, A. (2009). Hypoxia inducible factor-2alpha stabilization and maxi-K+ channel beta1-subunit gene repression by hypoxia in cardiac myocytes: Role in preconditioning. Circulation Research, 104, 1364–1372.
Arab, A., Kuemmerer, K., Wang, J., Bode, C., & Hehrlein, C. (2008). Oxygenated perfluorochemicals improve cell survival during reoxygenation by pacifying mitochondrial activity. Journal of Pharmacology and Experimental Therapeutics, 325, 417–424.
Jürgensen, J. S., Rosenberger, C., Wiesener, M. S., Warnecke, C., Hörstrup, J. H., Gräfe, M., et al. (2004). Persistent induction of HIF-1alpha and -2alpha in cardiomyocytes and stromal cells of ischemic myocardium. The FASEB Journal, 18, 1415–1417.
Meeson, A. P., Radford, N., Shelton, J. M., Mammen, P. P., DiMaio, J. M., Hutcheson, K., et al. (2001). Adaptive mechanisms that preserve cardiac function in mice without myoglobin. Circulation Research, 88, 713–720.
Shohet, R. V., & Garcia, J. A. (2007). Keeping the engine primed: HIF factors as key regulators of cardiac metabolism and angiogenesis during ischemia. Journal of Molecular Medicine (Berlin, Germany), 85, 1309–1315.
Kim, J. W., Tchernyshyov, I., Semenza, G. L., & Dang, C. V. (2006). HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism, 3, 177–185.
Rey, S., & Semenza, G. L. (2010). Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovascular Research, 86, 236–242.
Hinkel, R., Trenkwalder, T., & Kupatt, C. (2011). Gene therapy for ischemic heart disease. Expert Opinion on Biological Therapy, 11, 723–737.
Tyagi, S. C. (1997). Vasculogenesis and angiogenesis: Extracellular matrix remodeling in coronary collateral arteries and the ischemic heart. Journal of Cellular Biochemistry, 65, 388–394.
Metivier, F., Marchais, S. J., Guerin, A. P., Pannier, B., & London, G. M. (2000). Pathophysiology of anaemia: Focus on the heart and blood vessels. Nephrology, Dialysis, Transplantation, 15(Suppl 3), 14–18.
Frangogiannis, N. G., & Entman, M. L. (2005). Chemokines in myocardial ischemia. Trends in Cardiovascular Medicine, 15, 163–169.
Chevion, M., Jiang, Y., Har-El, R., Berenshtein, E., Uretzky, G., & Kitrossky, N. (1993). Copper and iron are mobilized following myocardial ischemia: Possible predictive criteria for tissue injury. Proceedings of the National Academy of Sciences of the United States of America, 90, 1102–1106.
Tapiero, H., Townsend, D. M., & Tew, K. D. (2003). Trace elements in human physiology and pathology. Copper. Biomedicine & Pharmacotherapy, 57, 386–398.
Davis, G. K., & Mertz, W. (1987). Trace elements in human and animal nutrition (pp. 301–364). New York: Academic Press.
Dutt, B., & Mills, C. F. (1960). Reproductive failure in rats due to copper deficiency. Journal of Comparative Pathology, 70, 120–125.
Fell, B. F., Farquharson, C., & Riddoch, G. I. (1987). Kidney lesions in copper-deficient rats. Journal of Comparative Pathology, 97, 187–196.
Goodman, J. R., Warshaw, J. B., & Dallman, P. R. (1970). Cardiac hypertrophy in rats with iron and copper deficiency: Quantitative contribution of mitochondrial enlargement. Pediatric Research, 4, 244–256.
Kopp, S. J., Klevay, L. M., & Feliksik, J. M. (1983). Physiological and metabolic characterization of a cardiomyopathy induced by chronic copper deficiency. The American Journal of Physiology, 245, H855–H866.
Elsherif, L., Ortines, R. V., Saari, J. T., & Kang, Y. J. (2003). Congestive heart failure in copper-deficient mice. Experimental Biology and Medicine (Maywood, NJ), 228, 811–817.
Wildman, R. E., Hopkins, R., Failla, M. L., & Medeiros, D. M. (1995). Marginal copper-restricted diets produce altered cardiac ultrastructure in the rat. Proceedings of the Society for Experimental Biology and Medicine, 210, 43–49.
Li, Y., Wang, L., Schuschke, D. A., Zhou, Z., Saari, J. T., & Kang, Y. J. (2005). Marginal dietary copper restriction induces cardiomyopathy in rats. The Journal of Nutrition, 135, 2130–2136.
Wester, P. O. (1965). Trace elements in human myocardial infarction determined by neutron activation analysis. Acta Medica Scandinavica, 178, 765–788.
Zama, N., & Towns, R. (1986). Cardiac copper, magnesium, and zinc in recent and old myocardial infarction. Biological Trace Element Research, 10, 201–208.
Chipperfield, B., & Chipperfield, J. R. (1978). Differences in metal content of the heart muscle in death from ischemic heart disease. American Heart Journal, 95, 732–737.
Prohaska, J. R. (1990). Biochemical changes in copper deficiency. The American Journal of Clinical Nutrition, 1, 452–461.
Medeiros, D. M., Davidson, J., & Jenkins, J. E. (1993). A unified perspective on copper deficiency and cardiomyopathy. Proceedings of the Society for Experimental Biology and Medicine, 203, 262–273.
Feng, W., Ye, F., Xue, W., Zhou, Z., & Kang, Y. J. (2009). Copper regulation of hypoxia-inducible factor-1 activity. Molecular Pharmacology, 75, 174–182.
Wang, G. L., Jiang, B. H., Rue, E. A., & Semenza, G. L. (1995). Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proceedings of the National Academy of Sciences of the United States of America, 92, 5510–5514.
Huang, L. E., Gu, J., Schau, M., & Bunn, H. F. (1998). Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proceedings of the National Academy of Sciences of the United States of America, 95, 7987–7992.
Wang, G. L., & Semenza, G. L. (1993). General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proceedings of the National Academy of Sciences of the United States of America, 90, 4304–4308.
Jaakkola, P., Mole, D. R., Tian, Y. M., Wilson, M. I., Gielbert, J., Gaskell, S. J., et al. (2001). Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science, 292, 468–472.
Ivan, M., Kondo, K., Yang, H., Kim, W., Valiando, J., Ohh, M., et al. (2001). HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science, 292, 464–468.
Maxwell, P. H., Wiesener, M. S., Chang, G. W., Clifford, S. C., Vaux, E. C., Cockman, M. E., et al. (1999). The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature, 399, 271–275.
Masson, N., Willam, C., Maxwell, P. H., Pugh, C. W., & Ratcliffe, P. J. (2001). Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. The EMBO Journal, 20, 5197–5206.
Ohh, M., Park, C. W., Ivan, M., Hoffman, M. A., Kim, T. Y., Huang, L. E., et al. (2000). Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nature Cell Biology, 2, 423–427.
Tanimoto, K., Makino, Y., Pereira, T., & Poellinger, L. (2000). Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. The EMBO Journal, 19, 4298–4309.
Hirsila, M., Koivunen, P., Xu, L., Seeley, T., Kivirikko, K. I., & Myllyharju, J. (2005). Effect of desferrioxamine and metals on the hydroxylases in the oxygen sensing pathway. FASEB Journal, 19, 1308–1310.
Ke, Q., Kluz, T., & Costa, M. (2005). Down-regulation of the expression of the FIH-1 and ARD-1 genes at the transcriptional level by nickel and cobalt in the human lung adenocarcinoma A549 cell line. International Journal of Environmental Research and Public Health, 2, 10–13.
Maxwell, P., & Salnikow, K. (2004). HIF-1: an oxygen and metal responsive transcription factor. Cancer Biology & Therapy, 3, 29–35.
Yuan, Y., Hilliard, G., Ferguson, T., & Millhorn, D. E. (2003). Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. The Journal of Biological Chemistry, 278, 15911–15916.
Mahon, P. C., Hirota, K., & Semenza, G. L. (2001). FIH-1: A novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes & Development, 15, 2675–2686.
Lando, D., Peet, D. J., Gorman, J. J., Whelan, D. A., Whitelaw, M. L., & Bruick, R. K. (2002). FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes & Development, 16, 1466–1471.
Lando, D., Peet, D. J., Whelan, D. A., Gorman, J. J., & Whitelaw, M. L. (2002). Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science, 295, 858–861.
McNeill, L. A., Hewitson, K. S., Claridge, T. D., Seibel, J. F., Horsfall, L. E., & Schofield, C. J. (2002). Hypoxia-inducible factor asparaginyl hydroxylase (FIH-1) catalyses hydroxylation at the beta-carbon of asparagine-803. The Biochemical Journal, 367, 571–575.
Freedman, S. J., Sun, Z. Y., Poy, F., Kung, A. L., Livingston, D. M., Wagner, G., et al. (2002). Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proceedings of the National Academy of Sciences of the United States of America, 99, 5367–5372.
Dames, S. A., Martinez-Yamout, M., De Guzman, R. N., Dyson, H. J., & Wright, P. E. (2002). Structural basis for Hif-1 alpha/CBP recognition in the cellular hypoxic response. Proceedings of the National Academy of Sciences of the United States of America, 99, 5271–5276.
Wang, G. L., & Semenza, G. L. (1993). Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: Implications for models of hypoxia signal transduction. Blood, 82, 3610–3615.
Jiang, B. H., Jiang, G., Zheng, J. Z., Lu, Z., Hunter, T., & Vogt, P. K. (2001). Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth & Differentiation, 12, 363–369.
Sandau, K. B., Faus, H. G., & Brüne, B. (2000). Induction of hypoxia-inducible-factor 1 by nitric oxide is mediated via the PI 3 K pathway. Biochemical and Biophysical Research Communications, 278, 263–267.
Treins, C., Giorgetti-Peraldi, S., Murdaca, J., Semenza, G. L., & Van Obberghen, E. (2002). Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. The Journal of Biological Chemistry, 277, 27975–27981.
Tramontano, A. F., Muniyappa, R., Black, A. D., Blendea, M. C., Cohen, I., Deng, L., et al. (2003). Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biochemical and Biophysical Research Communications, 308, 990–994.
Kim, C. H., Cho, Y. S., Chun, Y. S., Park, J. W., & Kim, M. S. (2002). Early expression of myocardial HIF-1alpha in response to mechanical stresses: Regulation by stretch-activated channels and the phosphatidylinositol 3-kinase signaling pathway. Circulation Research, 90, E25–E33.
Lee, S. H., Wolf, P. L., Escudero, R., Deutsch, R., Jamieson, S. W., & Thistlethwaite, P. A. (2000). Early expression of angiogenesis factors in acute myocardial ischemia and infarction. The New England Journal of Medicine, 342, 626–633.
Jurgensen, J. S., Rosenberger, C., Wiesener, M. S., Warnecke, C., Horstrup, J. H., Grafe, M., et al. (2004). Persistent induction of HIF-1alpha and -2alpha in cardiomyocytes and stromal cells of ischemic myocardium. FASEB Journal, 18, 1415–1417.
Kido, M., Du, L., Sullivan, C. C., Li, X., Deutsch, R., Jamieson, S. W., et al. (2005). Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. Journal of the American College of Cardiology, 46, 2116–2124.
Date, T., Mochizuki, S., Belanger, A. J., Yamakawa, M., Luo, Z., Vincent, K. A., et al. (2005). Expression of constitutively stable hybrid hypoxia-inducible factor-1alpha protects cultured rat cardiomyocytes against simulated ischemia-reperfusion injury. American Journal of Physiology. Cell Physiology, 288, C314–C320.
Kaelin, W. G., Jr, & Ratcliffe, P. J. (2008). Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Molecular Cell, 30, 393–402.
Berra, E., Benizri, E., Ginouves, A., Volmat, V., Roux, D., & Pouyssegur, J. (2003). HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. The EMBO Journal, 22, 4082–4090.
Minamishima, Y. A., Moslehi, J., Bardeesy, N., Cullen, D., Bronson, R. T., & Kaelin, W. G., Jr. (2008). Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood, 111, 3236–3244.
Appelhoff, R. J., Tian, Y. M., Raval, R. R., Turley, H., Harris, A. L., Pugh, C. W., et al. (2004). Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. The Journal of Biological Chemistry, 279, 38458–38465.
Minamishima, Y. A., Moslehi, J., Padera, R. F., Bronson, R. T., Liao, R., & Kaelin, W. G., Jr. (2009). A feedback loop involving the Phd3 prolyl hydroxylase tunes the mammalian hypoxic response in vivo. Molecular and Cellular Biology, 29, 5729–5741.
Huang, M., Chan, D. A., Jia, F., Xie, X., Li, Z., Hoyt, G., et al. (2008). Short hairpin RNA interference therapy for ischemic heart disease. Circulation, 118, S226–S233.
Lei, L., Mason, S., Liu, D., Huang, Y., Marks, C., Hickey, R., et al. (2008). Hypoxia-inducible factor-dependent degeneration, failure, and malignant transformation of the heart in the absence of the von Hippel-Lindau protein. Molecular and Cellular Biology, 28, 3790–3803.
Moslehi, J., Minamishima, Y. A., Shi, J., Neuberg, D., Charytan, D. M., Padera, R. F., et al. (2010). Loss of hypoxia-inducible factor prolyl hydroxylase activity in cardiomyocytes phenocopies ischemic cardiomyopathy. Circulation, 122, 1004–1016.
Bekeredjian, R., Walton, C. B., MacCannell, K. A., Ecker, J., Kruse, F., Outten, J. T., et al. (2010). Conditional HIF-1alpha expression produces a reversible cardiomyopathy. PLoS ONE, 5, e11693.
Giacca, M., & Zacchigna, S. (2012). VEGF gene therapy: Therapeutic angiogenesis in the clinic and beyond. Gene Therapy,. doi:10.1038.
Eibel, B., Rodrigues, C. G., Giusti, I. I., Nesralla, I. A., Prates, P. R., Sant’Anna, R. T., et al. (2011). Gene therapy for ischemic heart disease: Review of clinical trials. The Revista Brasileira de Cirurgia Cardiovascular, 26, 635–646.
Jeremy, J. Y., Shukla, N., Angelini, G. D., Day, A., Wan, I. Y., Talpahewa, S. P., et al. (2002). Sustained increases of plasma homocysteine, copper, and serum ceruloplasmin after coronary artery bypass grafting. The Annals of Thoracic Surgery, 74, 1553–1557.
Mansoor, M. A., Bergmark, C., Haswell, S. J., Savage, I. F., Evans, P. H., Berge, R. K., et al. (2000). Correlation between plasma total homocysteine and copper in patients with peripheral vascular disease. Clinical Chemistry, 46, 385–391.
Shukla, N., Angelini, G. D., & Jeremy, J. Y. (2007). Interactive effects of homocysteine and copper on angiogenesis in porcine isolated saphenous vein. The Annals of Thoracic Surgery, 84, 43–49.
Apostolova, M. D., Bontchev, P. R., Ivanova, B. B., Russell, W. R., Mehandjiev, D. R., Beattie, J. H., et al. (2003). Copper-homocysteine complexes and potential physiological actions. Journal of Inorganic Biochemistry, 95, 321–333.
Carrasco-Pozo, C., Alvarez-Lueje, A., Olea-Azar, C., Lopez-Alarcon, C., & Speisky, H. (2006). In vitro interaction between homocysteine and copper ions: Potential redox implications. Experimental Biology and Medicine (Maywood, NJ), 231, 1569–1575.
Emsley, A. M., Jeremy, J. Y., Gomes, G. N., Angelini, G. D., & Plane, F. (1999). Investigation of the inhibitory effects of homocysteine and copper on nitric oxide-mediated relaxation of rat isolated aorta. British Journal of Pharmacology, 126, 1034–1040.
Linnebank, M., Lutz, H., Jarre, E., Vielhaber, S., Noelker, C., Struys, E., et al. (2006). Binding of copper is a mechanism of homocysteine toxicity leading to COX deficiency and apoptosis in primary neurons, PC12 and SHSY-5Y cells. Neurobiology of Disease, 23, 725–730.
Martin, F., Linden, T., Katschinski, D. M., Oehme, F., Flamme, I., Mukhopadhyay, C. K., et al. (2005). Copper-dependent activation of hypoxia-inducible factor (HIF)-1: Implications for ceruloplasmin regulation. Blood, 105, 4613–4619.
van Heerden, D., Vosloo, A., & Nikinmaa, M. (2004). Effects of short-term copper exposure on gill structure, metallothionein and hypoxia-inducible factor-1alpha (HIF-1alpha) levels in rainbow trout (Oncorhynchus mykiss). Aquatic Toxicology, 69, 271–280.
Jiang, Y., Reynolds, C., Xiao, C., Feng, W., Zhou, Z., Rodriguez, W., et al. (2007). Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice. The Journal of Experimental Medicine, 204, 657–666.
Ziche, M., Jones, J., & Gullino, P. M. (1982). Role of prostaglandin E1 and copper in angiogenesis. Journal of the National Cancer Institute, 69, 475–482.
Harris, E. D. (2004). A requirement for copper in angiogenesis. Nutrition Reviews, 62, 60–64.
Lowndes, S. A., & Harris, A. L. (2005). The role of copper in tumour angiogenesis. Journal of Mammary Gland Biology and Neoplasia, 10, 299–310.
Nasulewicz, A., Mazur, A., & Opolski, A. (2004). Role of copper in tumour angiogenesis–clinical implications. Journal of Trace Elements in Medicine and Biology, 18, 1–8.
Goodman, V. L., Brewer, G. J., & Merajver, S. D. (2005). Control of copper status for cancer therapy. Current Cancer Drug Targets, 5, 543–549.
Sen, C. K., Khanna, S., Venojarvi, M., Trikha, P., Ellison, E. C., Hunt, T. K., et al. (2002). Copper-induced vascular endothelial growth factor expression and wound healing. American Journal of Physiology: Heart and Circulatory Physiology, 282, H1821–H1827.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, W., James Kang, Y. Ischemia-induced Copper Loss and Suppression of Angiogenesis in the Pathogenesis of Myocardial Infarction. Cardiovasc Toxicol 13, 1–8 (2013). https://doi.org/10.1007/s12012-012-9174-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-012-9174-y