Abstract
Lead (Pb) is a heavy metal which is abundant in the environment and known to cause neurotoxicity in children even at minute concentration. However, the trace elements calcium (Ca), magnesium (Mg), zinc (Zn) and iron (Fe) are essential to children due to its protective effect on neurodevelopment. The primary objective of this study was to assess the role of Pb and trace elements in the development of autism spectrum disorder (ASD) among preschool children. A total of 81 ASD children and 74 typically developed (TD) children aged between 3 and 6 years participated in the study. Self-administered online questionnaires were completed by the parents. A first-morning urine sample was collected in a sterile polyethene urine container and assayed for Pb, Ca, Mg, Zn and Fe using an inductively coupled plasma mass spectrometry (ICP-MS). Comparisons between groups revealed that the urinary Pb, Mg, Zn and Fe levels in ASD children were significantly lower than TD children. The odds of ASD reduced significantly by 5.0% and 23.0% with an increment of every 1.0 μg/dL urinary Zn and Fe, respectively. Post interaction analysis showed that the odds of ASD reduced significantly by 11.0% and 0.1% with an increment of every 1.0 μg/dL urinary Zn and Pb, respectively. A significantly lower urinary Pb level in ASD children than TD children may be due to their poor detoxifying mechanism. Also, the significantly lower urinary Zn and Fe levels in ASD children may augment the neurotoxic effect of Pb.
Similar content being viewed by others
Introduction
Lead (Pb) is a naturally occurring, non-ferrous, heavy metallic element found in the earth’s crust. Despite being one of the most toxic pollutants, Pb has been used worldwide in various industries and consumers’ products due to its malleability and corrosion resistance [1]. Human exposure to Pb is inevitable due to the current industrial revolution, rapid urbanisation and economic development. It is worthy to note that Pb has potent and irreversible health effects on human. For instance, Pb could become a potential cofactor, initiator or promoter in many diseases, even at an extremely low concentration [2]. Therefore, frequent human exposure to Pb in many countries worldwide has become a global environmental health concern. Humans can be exposed to Pb through oral ingestion or inhalation of Pb-contaminated soil and dust [3].
Young children are more vulnerable to Pb exposure than adults, as they have unique physiological characteristics. Compared to an adult, children’s digestive system has a higher oral intake [3] and Pb absorption rate [4] which may increase further when they are fasting or lack of essential trace elements [5, 6]. Additionally, mouthing behaviours in young children may expose them to Pb [7]. Young children often have the habit of pica (persistent and compulsive cravings to eat non-food items) due to their innate curiosity and inability to differentiate between non-food items and food [8]. Apart from the digestive system, exposure to Pb can easily harm the children’s immature and developing nervous system [9].
There are many potential sources of Pb in the environment, including Pb mining and smelting, Pb-related industries (especially batteries and electronics), indoor and outdoor Pb-based paint, water piping and solder, domestic products (e.g. colour pencils, crayons, toys painted with Pb-based paint, Pb-glazed ceramic, cigarette, leaded petrol, cosmetic products and traditional remedies) and hobbies involving Pb (e.g. fishing, painting and collecting electronic devices) [4, 10,11,12,13,14]. Workmen in the Pb-related industries could contribute to “take-home contamination” by carrying Pb dust on their clothes, footwear, skin and other personal attires to their home [4, 15]. Children living in urban cities may be exposed to other sources of Pb pollution (e.g. soil and dust) and emissions produced by anthropogenic activities (e.g. road traffic, industries, construction and demolition) [2, 16]. They spread through the air rapidly within the environment, contaminate the food chain and eventually enter the human bodies [17]. Other associated risk factors of childhood Pb exposure are parents’ education levels, social status, children’s behaviours, habits, diet and nutritional status [18].
Childhood Pb toxicity is a preventable environmental disease that has long-lasting adverse health and behavioural effects. Children exposed to Pb are prone to experience irreversible morphological and molecular alterations of the nervous system [19,20,21]. It has been well established that Pb toxicity has various adverse effects on the central neurological function. Consequently, these effects increase the risk of a broad spectrum of developmental delays, intellectual and behavioural deficits, hyperactivity, social withdrawal, gross and fine motor performance deficits and decreased intelligence quotient (IQ) [22,23,24,25,26,27]. Additionally, these effects have been associated with higher Pb concentrations within hours following birth [28].
An acute high concentration of Pb toxicity in children rarely occurs nowadays due to the government’s law and legislation’s effectiveness in many developed countries to regulate and control Pb [29]. Examples of these control measures are phasing out Pb in petrol and household paint and reducing industrial emission, water Pb and other sources [29]. However, a chronic low Pb toxicity concentration is equally worrisome and more common in children [30]. Numerous neurocognitive and neurobehavioural effects were observed in children with blood Pb levels (BLLs) below 10.0 μg/dL [31,32,33,34,35,36]. The United States Centres for Disease Control and Prevention (CDC) initially defined the elevated BLLs as 10.0 μg/dL or greater [37, 38]. However, the CDC later lowered the value to 5.0 μg/dL in 2012 [39, 40]. Regardless of the cut-off BLL concentration, no Pb level can be considered safe due to its adverse effects on the children’s progressive neurodevelopment [41, 42].
Autism spectrum disorder (ASD) describes a range of neurodevelopmental disorders, as stated in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5) [43]. It is characterised by abnormal social behaviour, disinterest in communication and interaction, language disorders, repetitive and obsessive behaviours and narrowly focussed rigid interests [44]. Since its first description in 1943 with a prevalence of 4.5 cases per 10,000 children [45], there has been a large increase in ASD prevalence [46,47,48,49,50]. The cause and aetiology of ASD remain controversial. With no current consensus, investigators from various biomedical fields are studying multiple possible causes of ASD.
The role of environmental factors (e.g. neurotoxic heavy metal exposure) on the development of ASD cannot be overlooked. The broad spectrum of ASD also suggests that the disease’s phenotype heterogeneity may result from exposure to certain environmental agents, instead of primarily due to genetic disorder [51]. The neurotoxicity and heavy metal (including Pb) exposure have been associated with the cause of neurodevelopmental disorders [52]. Previous studies reported that Pb could damage the developing human brain, causing a broad spectrum of neurodevelopmental disorders [25, 36, 53]. Depending on the level of dose toxicity, the disorders could range from overt clinical manifestation (high-dose toxicity) to subclinical dysfunction (low-dose chronic toxicity) [25, 36, 53].
Essential trace elements, which include calcium (Ca), magnesium (Mg), zinc (Zn) and iron (Fe), play important roles in children’s normal brain development, neurotransmitter synthesis catabolism, cellular metabolic process, metabolism relevant to neurotransmitters and motor development [54,55,56,57,58,59,60]. Therefore, altered levels of these elements and their imbalance may lead to dysfunction of neurotransmitters. Neurotransmitter dysfunction has been observed in many medical conditions and diseases, including neurological and behavioural disorders [61,62,63]. However, less is known about the role of these elements in ASD development. It has been suggested that the essential trace elements caused the excitatory and inhibitory synapses in ASD to dysfunction [64].
Mainly, Ca is crucial for neurodevelopment and may provide preventive and therapeutic effects towards ASD by regulating synapse development and function [65]. Ca often binds rapidly to target proteins and subsequently regulate Ca channel function, generating Ca signalling [66,67,68]. Mg is the fourth most regulatory cation in the body that modulates gamma-aminobutyric acid (GABA) signalling [69, 70]. Mg also activates the copper-zinc superoxide dismutase (CuZn-SOD) and nitric oxide released from cells [71]. The CuZn-SOD and nitric oxide are important in brain development and functional well-being [71]. Zn is required to scaffold Pro-SAP/Shank proteins related to excitatory synapses, where lower Zn concentrations have been associated with ASD [72]. On the other hand, Fe is an essential element for DNA synthesis, gene expression, myelination, neurotransmission and mitochondrial electron transport [73]. These functions are crucial for the central nervous system. Therefore, Fe deficiency impairs the neurotransmitter processes, myelin formation and energy metabolism in the brain, which was thought to cause behavioural and cognitive developmental delays in children [74, 75].
Regular consumption of dairy products and milk formulas is beneficial for children’s health [76]. The foods contain a high nutritional value that provides high amounts of micro-nutrients, mainly Ca [76]. However, excessive Ca intake may cause nephrolithiasis, milk-alkali syndrome and interfere with the absorption of other essential trace elements, such as Mg, Zn and Fe [77]. Mg is widely distributed in leafy vegetables, legumes, nuts, seeds, whole grains, animal foods and beverages [77]. Zn sources can be found in either protein-rich plant (e.g. cereal grains and legumes) and animal protein food [78]. Phytate (e.g. wholegrain cereals, legumes, nuts and seeds) and dietary Ca were known to inhibit Zn absorption, while protein enhances Zn absorption [77]. There are three main dietary sources of Fe: (i) breast milk (where Fe is bound to lactoferrin), (ii) haem Fe (meat, poultry and fish) and (iii) non-haem Fe (e.g. spinach, lentils, pumpkin seeds, beans, nuts and fortified cereals) [79]. The absorption of non-haem Fe depends on the total net effect of factors enhancing Fe absorption, such as ascorbic acid and organic acids (e.g. meat, chicken, fish and seafood), fermented vegetables and fermented soy sauces and factors inhibiting Fe absorption (e.g. phytates and inositol phosphates, Fe-binding polyphenols, Ca, soy proteins and vegetable proteins) [77].
Pb exposure is common in urban cities in Malaysia. In 2000, the prevalence of children with BLLs above 10.0 μg/dL was 11.7% in urban Kuala Lumpur [80]. In 2015, a study revealed that 27.0% of children in urban Malacca had blood Pb levels above 10.0 μg/dL [81]. The Federal Territory of Kuala Lumpur is Malaysia’s national capital and forms the nation’s most populous urban region. It is the city’s increasingly global orientation and its implications for the wider urban region [82]. The city’s total land area is 243.70 km2 (24,221.05 ha), which is a hundred percent urban area. It had a population of 1,556,200 people in 2005, with an average population density of 64 persons per hectare [83]. The population increased to 1,790,000 people in 2018 [84]. Kuala Lumpur’s rapid urbanisation increases environmental pollution and exposes children to neurotoxic heavy metals, especially Pb. Therefore, Kuala Lumpur was the most appropriate location to conduct this study. To the best of our knowledge, no study has assessed urinary Pb and essential trace elements in ASD among preschool children in Malaysia. Therefore, the primary objective of this study was to assess the role of Pb and essential trace elements in ASD development among preschool children in Malaysia.
Methodology
The current study protocol was approved by the National University of Malaysia (UKM) Research and Ethics Committee and the Medical Research and Ethics Committee of the Ministry of Health (MOH) Malaysia. All procedures were performed in accordance with the principles of the Declaration of Helsinki (1964) and later amendments. Participation was voluntary and informed written consents were obtained from the parents or legal caretakers before the study. This observational unmatched case-control study was conducted among the preschool children in Kuala Lumpur from January 15 until March 15, 2020. The study was completed before the first Movement Control Order (MCO) due to COVID-19 outbreak in Malaysia.
A total of 81 ASD children and 74 typically developed (TD) children were enrolled in the study. All children were Malaysian citizen aged between 3 and 6 years. Both group of children were randomly selected from the students’ name list with the schools’ permission. The ASD children were recruited from the national autism rehabilitation centre (GENIUS KURNIA), located in Sentul City, Kuala Lumpur. The centre is governed by the Ministry of Education (MOE) Malaysia. Clinical diagnosis of ASD was made by the paediatrician working in the government tertiary hospitals. The diagnosis was based on the DSM-5 criteria and the International Classification of Diseases-10 (ICD-10). The TD children in the control group were recruited from public preschools (age 4-6 years), namely TABIKA Department of Community Development (KEMAS) and public nurseries (age 2-4 years), namely TASKA KEMAS. The preschools and nurseries are located in Sentul City, Kuala Lumpur.
The two institutions were established and managed by the Early Childhood Education Division under the Ministry of Rural Development (MRD). The institutions were managed according to the National Preschool Standard Curriculum under the MOE, the National Early Childhood Care and Development Policy and the National PERMATA Curriculum [85]. The TD children were declared as “healthy”. They had no known characteristics of ASD, as verified by the paediatrician based on the Modified Checklist for Autism in Toddlers (M-CHAT) screening and the regular children health assessment during follow-up at 18 months and 36 months old. The following exclusion criteria were used for both groups: (i) congenital anomaly or syndrome, (ii) neurodevelopmental or neurobehavioural disorders, (iii) endocrine disorders, (iv) acute infectious, surgical and traumatic diseases and (v) currently on regular oral medications or infusion medications (chemotherapy) prescribed by specialists or on chelation therapy for heavy metal removal.
The researcher informed each participant’s parent (either father or mother) through phone calls, messages and emails to complete the self-administrated online questionnaire (Google Form). This method of gathering information online was preferred in this study due to several reasons: (i) easy access (through phone or computer), (ii) user-friendly, (iii) can be done at any time (especially for working parent who are busy during daytime), (iv) better data management (e.g. record keeping, confidentiality and data analysis) and (v) precautionary measure to the risk of COVID-19 transmission through closed contact during the COVID-19 outbreak in Malaysia. The researcher assisted the parent who had difficulty to complete the questionnaire through phone calls, messages and emails. The questionnaire was designed to elicit the information regarding the socio-demographic background of the parent and the child, the developmental milestone of the child, the risk factors that might indicate a predisposition to ASD (including pregnancy complications, preterm delivery, breastfeeding and family history of autism), the environmental exposure to Pb, the parental knowledge assessment on Pb and the dietary pattern of the child. The child’s anthropometric parameters (e.g. height and weight) were measured using calibrated digital weighing scales (Omron) that came with a height measurement stand. The researcher in the classroom recorded the measurements.
A first-morning urine sample was collected from each participant by the parent at home in a sterile polyethene urine container pre-treated with 20.0% nitric acid, HNO3 solution and rinsed twice with deionised water. Before the procedure, the parent was advised by the researcher regarding the correct technique of collecting urine sample: (i) the urine collection should be a clean catch, (ii) urine sample volume should range between 5.0 and 10.0 mL, (iii) the sterile polyethene urine container should not be contaminated with detergent, body soap or any foreign materials, (iv) the urine sample should not be added with water to avoid the dilutional effect and (v) proper closure of the urine container and the biohazard zip bag. The children were allowed to consume foods and drinks as usual. The urine samples were delivered by the parent to the researcher on the same day while they sent their children to the autism rehabilitation centre, preschool or nursery. The urine samples were labelled with code numbers and delivered to an accredited environmental laboratory, Faculty of Science and Technology, UKM, Bangi, Selangor within 24 h. The urine samples were stored at the temperature of −20.0 °C prior to the laboratory analysis.
In the laboratory, the urine samples were prepared by adding 1.0 mL of urine sample into 10.0 mL of 0.2% nitric acid (HNO3) solution with a ratio of 1:10. The preparation process was vital to allow the digestion process of organic matter in the urine sample. The prepared urine samples were then assayed for Pb and other essential trace elements (e.g. Ca, Mg, Zn and Fe) using the PerkinElmer SCIEX™ ELAN® 9000 inductively coupled plasma mass spectrometry (ICP-MS; PerkinElmer Inc., Shelton, CT 06484, USA). The detection limits using this operating system for each element were as follows: Pb 1.0–10.0 part per trillion (ppt), Ca 10.0–100.0 ppt, Mg 1.0–10.0 ppt, Zn 1.0–10.0 ppt and Fe 1.0–10.0 ppt. The system was calibrated using standard solutions prepared by the Universal Data Acquisition Standards Kit (Perkin Elmer Inc., Shelton, CT 06484, USA). Internal online standardisation was performed to assess the difference in matrix viscosity using 10.0 μg/L solutions of yttrium and rhodium Pure Single-Element Standard (Perkin Elmer Inc., Shelton, CT 06484, USA) [86].
The dataset from the questionnaire and the laboratory ICP-MS results were analysed using the IBM Statistical Package for Social Sciences (SPSS) software (version 22, IBM, Chicago, IL, USA). Prior to the statistical analysis, the data normality was explored graphically (based on histogram and Q-Q plot) and statistically (based on skewness, kurtosis and Shapiro-Wilks/Kolmogorov-Smirnov statistics). Frequency and percentage were calculated for each participant’s demographic parameters. The group differences in Pb levels (mean ± standard deviation) and other essential trace elements (Ca, Mg, Zn and Fe) were assessed either using the Student’s t-test (for normal distribution) or Mann-Whitney U test (for non-normal distribution). The potential associated risk factors and confounders (quantitative variables) were also assessed using one of the two methods mentioned above. The median, interquartile range (IQR), minimum and maximum of the analysed elements were also used as descriptive statistics. The categorical variables were calculated using the Chi-square test and presented in frequency and raw percentage. The magnitude of the correlation between element’s concentrations in the urine was analysed using the Pearson correlation test (for normal distribution) and by the Spearman rank correlation test (for non-normal distribution test). The receiver operating characteristic (ROC) analysis was performed as a comprehensive tool to assess the measured elements’ accuracy and choose the elements’ cut-off points. Simple and multiple logistic regression analyses were performed to assess the factors (independent variables included heavy metal, Pb) associated with ASD. The final prediction model allowed for an estimation of the effect (odds ratio) of the factors. A p-value of less than 0.05 was regarded as statistically significant in this study.
Results
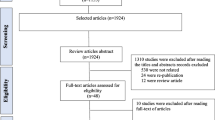
Figure 1 shows a comparison of the urinary Pb concentration level and essential trace elements (Ca, Mg, Zn and Fe) between ASD children and TD children according to children’s age (in months). The data of the concentration level of the elements was not normally distributed. Therefore, a non-parametric test was used to analyse the essential trace element data. The outliers were retained, and log transformation was not performed to preserve the true findings. The general characteristics of the ASD children and TD children are shown in Table 1.
A total of 155 preschool children (81 ASD children and 74 TD children) participated in the study. The male-to-female ratio was approximately 5:1 for ASD children and 1:1 for TD children (p < 0.001). Most of the children in both groups were Malays. For the ASD children and TD children, the Malay percentage were 75.0% and 94.6%, respectively (p = 0.003). Despite having ASD, approximately 17.3% of ASD children were able to talk at the age of 3 years old. In contrast, all TD children (100.0%) were able to speak at the age of 3 years old since the ability to speak was one of the control group’s inclusion criteria.
The parents of the ASD children were approximately 1 year older than the TD children’s parents (p = 0.047). Most parents in each group aged more than 30 years (p < 0.001), indicating that the parental age’s proxy during conception was appropriate. More than half of the ASD children were the first child in the family, while about 30.0% of the TD children were a subsequent child in the family (p < 0.001). Most parents had secondary education (p < 0.001) and were from B40 income group (less than RM5000.00/month) (p < 0.001).
A majority of the ASD children lived outside Kuala Lumpur, mainly in Selangor. On the other hand, most TD children lived in Kuala Lumpur (p < 0.001). Most ASD children were born in the government hospital (51.9%), outside Kuala Lumpur (63.0%) (p < 0.001). Meanwhile, most TD children were born in public hospitals (85.1%) in Kuala Lumpur (56.8%) (p = 0.014). Most ASD children stayed in middle-range houses (32.1% terrace houses and 32.1% condominiums), while most TD children stayed in flat houses (54.1%). The houses were older in the ASD group (22.28 ± 14.62 years), compared to TD children (18.28 ± 9.78 years) (p = 0.045).
Most of the parents were non-smoker (65.4% in the ASD children group, and 81.1% in the TD children group) (p = 0.011). The parents claimed no risk of Pb exposure at their workplace (93.8% in the ASD children group and 82.4% in the TD children group) (p = 0.027). There were no significant differences between the groups for parent’s gender, children’s age, children’s immunisation status, children’s BMI, ASD in family, obstetric risk factors, house location (nearby the main road, factory and construction site) and source of drinking water (p > 0.05). However, there was a significant mean difference of urinary Pb level between groups for the place of birth (p = 0.046) and the duration of breastfeeding (p = 0.013) among ASD children, and proximity of the house to the construction site (p = 0.038) among TD children.
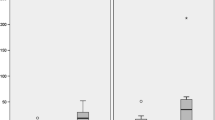
As shown in Fig. 2 and Table 2, laboratory analysis of urinary Pb and essential trace elements revealed statistically significant differences between the groups (p < 0.05), except Ca (p = 0.096). Surprisingly, the urinary Pb levels were significantly lower in ASD children (mean 0.26 ± 0.31 μg/dL) compared to TD children (mean 0.58 ± 0.41 μg/dL) (p < 0.05). Urinary Mg, Zn and Fe were also lower in ASD children than TD children. Further assessment was done for all the male children and children aged above 4 years. The result revealed a similar trend for all elements. However, there was a marked difference in the mean level of elements between the groups. For children aged 4 years and below, all elements were higher in the ASD children group than the TD children group. However, the finding was not statistically significant (p > 0.05), except Ca (p = 0.002).
As shown in Table 3, the overall correlation analysis demonstrated a significant positive association between the urinary Pb and the essential trace elements, except Ca (r = −0.01, p > 0.05). The association levels shown on this correlation ranged from very weak (Pb × Mg, r = 0.19) to moderate (Pb × Zn, r = 0.44). The correlation between the essential trace elements revealed a significant positive association ranging from weak (Ca × Zn, r = 0.25) to a very strong association (Ca × Fe, r = 0.87). A similar trend was found in all the male children (n = 107) and those aged above 4 years (n = 140). In the ASD and TD children groups, the correlation analysis revealed a non-significant positive association between the urinary Pb and the essential trace elements (p > 0.05), except for Pb × Zn (r = 0.26, p > 0.05) in male ASD children (n = 68).
The overall correlation between the essential trace elements revealed a significant positive association, with the association level ranging from weak (Ca × Zn, r = 0.25) to a very strong association (Ca × Fe, r = 0.87). A similar trend was found in all the male children (n = 107), all children aged above 4 years old (n = 140), all ASD children (n = 81), male ASD children (n = 68) and ASD children aged above 4 years old (n = 76). The correlation between essential trace elements showed moderate to a very good association in all TD children (n = 74) and TD children aged above 4 years old (n = 64), and excellent association in male TD children (n = 39). The correlation between urinary Ca and Fe produced persistent good to excellent association among different groups of participants (correlation coefficient, r from 0.64 to 0.97). Despite the non-significant findings, the correlation between urinary Pb and Ca showed a negative very weak to weak associations among all children (n = 155), male ASD children (n = 68), ASD children aged 4 years and below (n = 5), female TD children (n = 35) and TD children aged 4 years and below (n = 10).
Table 4 demonstrates each urinary element’s cut-off point among the 155 children using the ROC curve analysis. The area under the curves for urinary Pb and Zn showed the significant value closest to one (0.84 and 0.81, respectively). On the other hand, the urinary Ca, Mg and Fe showed the value closest to 0.5 (0.57, 0.59 and 0.65, respectively). The cut-off point for all elements was within the standard reference level.
Table 5 shows the multiple logistic regression analysis results of potential associated factors of ASD in both groups. Parental education, the children’s ethnicity, the children’s gender and parental smoking status were identified as significant associated factors of ASD. Parents with tertiary education had 26 times the odds of having ASD child compared to parents with secondary education (OR = 26.15, 95% CI 7.10, 96.38, p < 0.001). The odds of ASD in non-Malay children were 7.5 times higher than Malay children (OR = 7.52, 95% CI 1.62, 34.85, p = 0.010). The odds of ASD in male children were 8.5 times higher than for female children (OR = 8.52, 95% CI 2.76, 26.28, p < 0.001). An ex-smoker parent had 25 times of having ASD child compared to a non-smoker parent (OR = 25.29, 95% CI 4.03, 158.68, p = 0.001).
Table 6 shows the multiple logistic regression analysis results of urinary Pb and essential trace elements. The interactions between urinary elements Pb and Ca, Pb and Mg, Pb and Zn and Pb and Fe were significant. Therefore, these interactions were included in the multiple logistic regression analysis. The odds of ASD significantly reduced by 0.1% with increased of every 1.0 μg/dL urinary Pb after further interaction analysis (OR = 0.001, 95% CI 0.00, 0.89, p = 0.046). The odds of ASD significantly increased by 24.0% with increased of every 1.0 μg/dL urinary Ca (OR = 1.24, 95% CI 1.13, 1.36, p < 0.001). After further interaction analysis, the odds of ASD increased by only 4.0% with increased of every 1.0 μg/dL urinary Ca. However, the result was non-significant (OR = 1.24, 95% CI 1.13, 1.36, p < 0.001). An increment of every 1.0 μg/dL urinary Zn reduced the odds of ASD by 5.0% (OR = 0.95, 95% CI 0.91, 0.99, p = 0.008). However, after further interaction analysis, the odds of ASD significantly reduced by 11.0% with increment of every 1.0 μg/dL urinary Zn (OR = 0.89, 95% CI 0.83, 0.93, p = 0.001). An increment of every 1.0 μg/dL urinary Fe reduced the odds of ASD by 23.0% (OR = 0.77, 95% CI 0.69, 0.87, p <0.001). The odds of ASD reduced by only 5.0% with increment of every 1.0 μg/dL urinary Fe. However, the result was non-significant (OR = 0.95, 95% CI 0.73, 1.24, p = 0.698).
Discussion
Urinary Pb as a Biomonitor to Assess Body Burden of Pb
The determination of Pb in urine is considered to reflect the absorbed Pb that has diffused from plasma and is excreted through the kidneys, which accounts for about two-thirds of total elimination [87, 88]. The urinary Pb is understood to reflect Pb exposure within the last few days to weeks [88, 89]. It also explains possible long-term Pb exposure [87, 90]. The absorbed Pb from blood is deposited into calcified tissues (e.g. bone) and can be stored for decades [91, 92]. Pb is slowly released from the calcified tissue based on the bone turnover rates, either from a compact structure (slow turnover) or from a trabecular structure (rapid turnover) [92], depending on age or intensity of exposure [93]. In addition, the continuous growth of young children indicates constant bone remodelling for skeletal development. The constant bone remodelling contributes to endogenous contamination where stored Pb in the bone is continuously released into the plasma [92, 94]. The cortical bone contributes about more than a twofold concentration of Pb excreted in the urine per day compared to trabecular bone [95].
The 24-h urine collection method has its limitations, although it has been used frequently in many clinical studies. The limitations are the method is inconvenient and may potentially contaminate the urine samples with heavy metals [96]. Several investigators suggested that the short duration of urine collection can provide sufficient information about Pb excretion [97]. For instance, Gulson et al. revealed an extremely good correlation between blood-urine pairs for isotopic Pb composition, indicating that urine can serve as an alternative for blood especially in new born infants and young children [96]. Fukui et al. suggested urine Pb to be a good alternative to blood Pb measurement on a group basis, in which the urine Pb was not adjusted by creatinine concentration [98]. In this study, the spot collection of urine for Pb measurement was chosen as it is the commonest and the most preferable biological sample in the biomonitoring studies involving children. Non-invasive samples (e.g. urine) were collected instead of invasive samples (e.g. blood) as the clinical procedures are difficult to perform on young children and create parental anxiety, which could lead to less participation and potentially result in selection bias [99, 100].
The Concentration of Pb Below Elevated Level
The urinary Pb concentration for both groups in this study was below the elevated level of 5.0 μg/dL. The highest recorded concentration level of urinary Pb was 2.5 μg/dL. Out of the 155 participants, most children (90.0%) had urinary Pb level below 1.0 μg/dL (n = 135). Previously accumulated data (since the early 1990s) have provided sufficient evidence about the toxic effects of Pb occurring at low concentration level [101]. Since children are more vulnerable to Pb exposure and more likely to suffer from neurodevelopmental deficits, the importance of adverse health effects in young children cannot be underestimated [102]. Previous cohort studies have shown significant inverse associations among most or all children with BLLs below 10.0 μg/dL [36, 103, 104] and as low as 1.0–2.0 μg/dL in other cohort studies [105,106,107]. The available evidence suggests that the mean BLLs range between 2.0 and 4.0 μg/dL in the US and European countries [108]. Our study demonstrated a significant cut-off point of 0.25 μg/dL for urinary Pb from the ROC curve analysis, indicating a possibility of a neurotoxic effect of Pb at this level. However, the minimum level to cause the neurological effect, especially in young children, cannot be concluded from the present findings.
The low urinary Pb level in all children in this study could reflect that the Pb exposure in urban Kuala Lumpur has improved. Previous studies done in urban Kuala Lumpur showed a reducing trend of Pb level among children from 5.26 μg/dL (BLLs) in 2000 [80] to 3.40 μg/dL (BLLs) in 2007 [109]. In the current study, the Pb level further dropped to 0.42 μg/dL (urinary Pb). This comparison is valid, although different biological samples were used in those studies because the Pb concentrations in urine are generally lower by a minimum factor of 10 compared to Pb in the blood [88].
The reasons for the low Pb levels in children in Malaysia over the two decades could be due to the Malaysian government’s action to phase out Pb from gasoline since early 1998 [110]. Consequently, Pb concentration in the air reduced greatly from 1990 to 2004 [111]. The Malaysian government also formulated a series of policies, including the latest National Automotive Policy (NAP) 2020 [112], to encourage the use of alternative vehicles. Examples of these vehicles are battery electric vehicles (BEVs) and public transportation (e.g. electric bus, monorail and electric train). Additionally, Malaysia planned to regulate heavy metals (including Pb) in the ceramic ware since 2014. However, in 2020, Malaysia notified the World Trade Organization (WTO) regarding the maximum release amounts for Pb for cookware during testing to a new standard level of 0.5 mg/L [113]. This new standard supersedes the 13th schedule of the Food Regulations 1985, which stated that Pb in the leachate from packaging, appliance containers and vessels used for cooking should not exceed 2.0 mg/L [114].
The Ministry of Domestic Trade, Co-operatives and Consumerism Malaysia (MDTCC) regulates mandatory safety standards for toys intended for children aged below 14 years old. The maximum acceptable migration of Pb in paint shall not be more than 90.0 ppm [115]. Malaysia also introduced local legislative frameworks to manage the country’s overall e-waste sector. These frameworks include generation, movement, recycling and disposal based on laws and legislations relevant to scheduled waste and e-waste management in Malaysia. These laws and legislations include the Environmental Quality Act (EQA) 1974, Environmental Quality (Prescribed Premises) (Scheduled Wastes Treatment and Disposal Facilities) Regulations 1989, Environmental Quality (Prescribed Premises) (Scheduled Wastes Treatment and Disposal Facilities) Order 1989, Environmental Quality (Scheduled Wastes) Regulations 2005, Customs (Prohibition of Import) Order 2012 and Customs (Prohibition of Export) Order 2017 [116].
Reduced Urinary Pb Concentration in ASD Children
Our results do not support an early hypothesis that high Pb in the urine was associated with ASD among preschool children in urban Kuala Lumpur. Instead, Pb concentration was significantly lower (p < 0.05) in the ASD children’s urine samples than TD children (0.26 μg/dL for ASD children versus 0.58 μg/dL for TD children). When we adjusted for a potential confounding factor, such as age and gender (as shown in Table 2), there was no sufficient evidence to indicate a higher urinary Pb concentration in ASD children than TD children.
However, our univariable results are consistent with several other studies since the early 1980s until 2020. These studies reported lower urinary Pb levels in ASD children than TD children, as listed in Table 7. For example, Marlowe et al. reported significantly lower Pb level in the hair sample of ASD children (mean 6.28 ± 2.12 ppm) compared to race-matched and social class-matched TD children (mean 6.66 ± 2.49 ppm) [118]. In Japan, Yasuda et al. reported significantly lower Pb level in the hair sample of ASD children (mean 0.39 ± 0.23 ppb) compared to age-matched and gender-matched TD children (mean 0.89 ± 0.50 ppb) [119]. In Turkey, Yorbik et al. reported significantly lower Pb level in the urine sample of ASD children (mean 1.19 μg/g creatinine) compared to unmatched TD children (mean 4.63 μg/g creatinine) [122]. In Saudi Arabia, Alabdali et al. reported significantly lower Pb level in the blood sample of ASD children (mean 4.73 μg/dL) compared to age-matched and gender-matched TD children (mean 6.79 μg/dL) [126]. In Jamaica, Rahbar et al. reported significantly lower Pb level in the blood sample of ASD children (mean 2.25 μg/dL) compared to age-matched and gender-matched TD children (mean 2.73 μg/dL) [127]. Five years later, the same author (Rahbar et al.) reported significantly lower Pb level in the blood sample of ASD children (geometric mean 1.92 μg/dL) compared to age-matched and gender-matched TD children (geometric mean 2.34 μg/dL) [131].
Poor Excretory Mechanism of Pb in ASD Children
The finding of the current study also supports the value concept that the ASD children might have a decreased ability to excrete the heavy metals (including Pb) and may be considered poor detoxifiers relatively to TD children, as supported by the previous evidence [132, 133]. The decreased ability to excrete the heavy metals may lead to a higher body burden and subsequent neurological damage [121, 134]. The reason ASD children had difficulty to excrete heavy metals (including Pb) remains unclear and not well explained. However, it is postulated that the poor mechanism of Pb excretion could be explained by plausible hypotheses which are the presence of specific antioxidant in the body and competitive mechanism of Ca towards Pb during excretion.
Oxidative stress in response to environmental insults plays a role in essentially every human disease. It is also presumed to be involved in the aetiology of ASD, in which decreased antioxidant capacity and increased oxidative stress in ASD can lead to neural structure damage and impair neural functioning [135, 136]. Endogenous thiols, such as glutathione (GSH), L-cysteine, N-acetyl cysteine (NAC), taurine and melatonin, are examples of important antioxidants. These antioxidants can reduce metal availability, decrease damage to the organ cells and biological macromolecules and promote detoxification. The antioxidants work through various action mechanisms, such as scavenging free radicals, interrupting radical chain reactions and forming stable complexes with heavy metals, including Pb [121, 137]. Therefore, the decreased level of antioxidants in the body of ASD children will promote sequestration of heavy metals in the children’s brain and subsequently excrete lower concentration of heavy metals in the urine [121].
Role of Essential Trace Element Towards Pb
Besides urinary Pb, the concentration levels of certain essential trace elements were significantly lower in ASD children than TD children; urinary Zn (39.81 μg/dL for ASD children versus 88.88 μg/dL for TD children) and urinary Fe (34.69 μg/dL for ASD children versus 58.32 μg/dL for TD children). The concentration level of urinary Mg was also lower in ASD children than TD children, but the result was non-significant. However, when we adjusted the age and gender, the mean difference of urinary Mg appeared to be significant in children aged above 4 years (102.34 μg/dL for ASD children versus 140.45 μg/dL for TD children) and male children (106.00 μg/dL for ASD children versus 139.96 μg/dL for TD children). In contrast, the result showed that the urinary Ca was significantly higher in ASD children than TD children aged 4 years and below (107.95 μg/dL for ASD children versus 34.18 μg/dL for TD children).
The univariable findings of essential trace elements in this study are consistent with the previous studies for Mg, Zn and Fe. For instance, Skalny et al. demonstrated that the Mg concentration in the hair (17.91 μg/g for ASD children versus 18.84 μg/g for TD children) and urine (108.59 μg/mL for ASD children versus 118.51 μg/mL for TD children) of ASD children was lower than the unmatched TD control. However, the findings were non-significant [138]. Priya et al. demonstrated that the Mg concentrations in hair of low functioning autism (LFA) (mean 174.02 ± 20.88 μg/g), medium functioning autism (MFA) (mean 202.21± 24.26 μg/g) and high functioning autism (HFA) (mean 236.31 ± 28.35 μg/g) children were significantly lower than the control group (mean 454.36 ± 54.52 μg/g). This finding indicated that the severity of ASD increases with the reducing Mg concentration level in the hair [139]. Strambi et al. demonstrated significantly lower plasma Mg level in ASD children (mean 2.27 ± 0.33 mg/100 mL) compared to unmatched healthy children (mean 2.51 ± 0.14 mg/100 mL) [70]. A systematic review and meta-analysis study reported significantly lower Mg levels in hair (after removal of an outlier study with effect size of −0.612, z-value = 2.68, p = 0.007) and serum (effect size of −0.105, z-value = 5.88, p < 0.001) of ASD children than healthy controls [140].
As for Zn, Priya et al. demonstrated the significantly lower concentration of Zn in the hair of LFA children (mean 130.46 ± 15.65 μg/g) than the control group (mean 171.68 ± 20.60 μg/g) [139]. Li et al. reported significantly lower concentration of Zn in the serum of ASD children (mean 78.70 ± 7.00 ng/mL) compared to age-matched and gender-matched healthy controls (mean 87.70 ± 8.70 ng/mL) [141]. Saghazadeh et al. reported a significant effect size of −0.361 (z-value = 2.31, p = 0.021), indicating that the ASD patients (n = 513) had lower blood Zn levels than controls (n = 333), after excluding two outlier studies. Further sensitivity hair sample analyses indicated that Asian patients with ASD (n = 236) had lower Zn levels in the hair (standardised mean difference (SMD) = −1.493, p = 0.002) than their Asian counterparts (n = 306), after excluding an outlier study [140].
As for Fe, Lubkowska et al. demonstrated that the Fe concentration in hair of ASD children (mean 9.02 ± 4.62 μg/g) was significantly lower than age-matched healthy controls (mean 10.05 ± 2.92 μg/g) [142]. Additionally, Saghazadeh et al. reported a significant effect size of −1.410 (z-value = 2.38, p = 0.017), indicating that the Fe levels in the hair of ASD children were lower than healthy children, after excluding an outlier study [140].
As for Ca, our univariate analysis contradicted with other trace elements (Mg, Zn and Fe). However, a recent study reported a similar finding, whereby the higher concentration of Ca was found in the serum of ASD children (median 109.16, 25–75 percentiles 103.55–113.5) than age-matched and gender-matched neurotypical children (median 106.71, 25–75 percentiles 103.82–112.3) [143]. However, the result was non-significant.
From the regression analysis of urinary trace elements, urinary Zn appeared to be a protective factor of ASD (OR = 0.95, 95% CI 0.91, 0.99, p = 0.008). The protective effect exerted by the urinary Zn was significantly increased after further interaction analysis (OR = 0.89, 95% CI 0.83, 0.95, p = 0.001). The urinary Fe was found to exert a protective effect towards ASD (OR = 0.77, 95% CI 0.69, 0.87, p < 0.001). However, the protective effect of urinary Fe was reduced and non-significant after further interaction analysis (OR = 0.95, 95% CI 0.73, 1.24, p = 0.698). These findings signified that the presence of essential trace elements in the body, especially Zn and Fe, is crucial to counteract Pb’s neurotoxic effect.
The essential trace elements (e.g. Mg, Zn and Fe) play a vital role as antioxidant agents, whereby the presence of these elements in the body helps to prevent redistribution and accumulation of metal in tissues, reduces metal availability, decreases toxicity, stabilises cell membranes and decreases damage to biological macromolecules [137]. These elements also decrease teratogenic toxicity by decreasing the replacement of essential ions, forming insoluble metal-mineral complexes and producing metal-binding proteins (MT) [137]. Essential trace elements also decrease gastrointestinal absorption of heavy metals and decrease its distribution through competitive absorption mechanism [137]. However, our findings failed to support the theory. The correlation between urinary Pb and essential trace elements showed a significantly positive very weak to moderate correlation coefficient (r-value ranged from 0.19 to 0.44), except for the correlation between urinary Pb and Ca, which showed a non-significantly negative very weak correlation (p > 0.05).
Assessment of Other Associated Factors of ASD
Several associated factors of ASD were identified. These factors were ethnicity, parental education, children’s gender and parental smoking status. Our finding showed that parents with tertiary education had 26 times the odds of having an ASD child than parents with secondary education (OR = 26.15, 95% CI 7.10, 96.38, p < 0.001). This finding was supported by Eow et al., where the odds of having an ASD child among mother with tertiary education was 3.5 times higher compared to mother with secondary education or lower (OR = 3.47, 95% CI 1.00, 5.94) [144].
In terms of ethnicity, the proportion of non-Malay was low in the ASD children group (n = 18/63 (22.2%)) and TD children group (n = 4/70 (5.4%)). However, ethnicity (i.e. non-Malay) contributed a significant risk factor towards ASD (OR =7.52, 95% CI 1.62, 34.85, p = 0.010). A study reported that the non-Malay children had about 4.5 times the odds of developing ASD compared to Malay children (OR = 4.52, 95% CI 2.10, 6.94) [144].
As for gender, the male-to-female ratio of ASD children was 5:1. This ratio is higher than the previously reported ratio of 4:1 [145] and 3:1 [146, 147]. Therefore, male gender was a significant risk factor for ASD (OR = 8.52, 95% CI 2.76, 26.28, p < 0.001).
Lastly, the findings showed that parents (either father or mother) who were an ex-smoker had higher odds of having an ASD child than non-smoking parents (OR = 25.29, 95% CI 4.03, 158.68, p = 0.001). However, the finding was non-significant for parents who were an active smoker, indicating that the exposure towards heavy metals (including Pb) might occur during prenatal and antenatal periods. The parents’ decision to stop smoking might be influenced by their children being diagnosed as ASD. The risk of getting an ASD child is still high (OR = 3.53, 95% CI 1.30, 9.56) although the pregnant mother is only a second-hand smoker (mostly related to smoking husband or spouse) [148].
Recommendation
This study focussed on the prevention strategies for ASD, particularly from environmental health and nutrition perspectives. Since there is no safe Pb level, young children should not be exposed to Pb. If they are still being exposed to Pb, the exposure level should be minimised. Top stakeholders (i.e. government) should initiate and improve preventive measures by implementing the relevant laws and legislations. The current regulations stated that the standard Pb level should be frequently revised and amended when necessary. The enforcement should be strengthened to control and monitor Pb-based product manufacturing (e.g. paint, ceramic ware, toys, electric and electronic devices).
We also recommend the government, especially the MOH, to initiate the first national Pb screening programme among newborn babies and preschool children. This practice has been done in the USA for a few decades ago. The programme could probably start by identifying the high-risk group of babies and young children through a risk-based assessment of the family’s socio-demographic background. Additionally, the prevalence of childhood exposure at the national level and burden of environmental-related disease could be identified and analysed for further action by the relevant stakeholders. The MOH could also decide on the different types of samples for biomonitoring depending on the objectives (short-term or long-term exposure monitoring) and laboratory analysis costs. At the family level, parents or caretakers should have adequate knowledge about the health effect of toxic environmental elements to minimise Pb exposure to their children. Parental knowledge about Pb exposure should be improved via various health educations, either from mass media, electronic social media and health care centre (e.g. health clinics and hospitals).
In terms of nutrition, we recommend the parents or caretakers to provide adequate essential trace elements to their children. As mentioned in this article, the essential trace elements provide many benefits to the children’s body when consumed adequately. According to the Recommended Nutrient Intakes (RNI) for Malaysia 2017 by the National Coordinating Committee on Food and Nutrition, MOH [77], the recommended Ca intake for children age 1–3 years and 4–6 years is 700.0 mg per day and 1000.0 mg per day, respectively. For Mg, the recommended intake for children age 1–3 years old and 4–8 years old is 80.0 mg per day and 130.0 mg per day, respectively. On the other hand, the recommended Zn intake for children age 1–3 years old and 4–6 years old is 4.2 mg per day and 5.2 mg per day, respectively. Lastly, the recommended Fe (with 10.0% bioavailability) intake for children age 1–6 years old is 6.0 mg per day, whereas the recommended Fe (with 15.0% bioavailability) intake for 1–6 years old is 4.0 mg per day.
Limitations
The results of the current study should be interpreted carefully. Pb and essential trace elements were investigated only in the urine samples, which may not fully explain a complex pathological mechanism occurring in the brain due to these elements. Moreover, the relatively inconsistent results in the Pb levels of ASD children may be attributed to the heterogeneity (spectrum) of ASD, subjects’ diverse geographic locations or methodological differences. Nevertheless, these results indicated that further studies are warranted to investigate the possible role of Pb and other heavy metals in ASD.
Conclusion
The Pb concentration level in the urine samples of both groups was below the CDC’s elevated level. The study also found that the Pb concentration level was significantly lower in ASD children than TD children. The low Pb concentration level may be due to the poor detoxifying mechanism, which retains more Pb in the body while excreting less Pb in the urine. In addition, significantly lower concentration of the essential trace elements, namely urinary Mg, Zn and Fe, may augment the neurotoxic effect of Pb in ASD children. These findings imply the importance of essential trace elements in protecting the children’s central nervous system. Prevention strategies should be consistent and involve stakeholders and parents’ participation to ensure the children’s exposure towards Pb is minimised. Prevention strategies are crucial to provide optimum nutrition to reduce the occurrence and the progress of ASD among preschool children.
Data Availability
All data generated or analysed during this study are included in this published article. The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
Li M-M, Gao Z-Y, Dong C-Y, Wu M-Q, Yan J, Cao J, Ma W-J, Wang J, Gong Y-L, Xu J (2020) Contemporary blood lead levels of children aged 0–84 months in China: a national cross-sectional study. Environ Int 134:105288. https://doi.org/10.1016/j.envint.2019.105288
Christoforidis A, Stamatis N (2009) Heavy metal contamination in street dust and roadside soil along the major national road in Kavala’s region, Greece. Geoderma 151(3-4):257–263. https://doi.org/10.1016/j.geoderma.2009.04.016
Bose-O’Reilly S, Yabe J, Makumba J, Schutzmeier P, Ericson B, Caravanos J (2018) Lead intoxicated children in Kabwe, Zambia. Environ Res 165:420–424. https://doi.org/10.1016/j.envres.2017.10.024
Hauptman M, Bruccoleri R, Woolf AD (2017) An update on childhood lead poisoning. Clin Pediatr Emerg Med 18(3):181–192. https://doi.org/10.1016/j.cpem.2017.07.010
Ab Latif Wani AA, Usmani JA (2015) Lead toxicity: a review. Interdiscip Toxicol 8(2):55–64. https://doi.org/10.1515/intox-2015-0009
Li Y, Qin J, Wei X, Li C, Wang J, Jiang M, Liang X, Xia T, Zhang Z (2016) The risk factors of child lead poisoning in China: a meta-analysis. Int J Environ Res Public Health 13(3):296. https://doi.org/10.3390/ijerph13030296
Lanphear BP, Roghmann KJ (1997) Pathways of lead exposure in urban children. Environ Res 74(1):67–73. https://doi.org/10.1006/enrs.1997.3726
World Health Organization (2019) Newsroom. Fact Sheets. Detail. Lead poisoning and health. https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health. Accessed 24 December 2020
Needleman H (2004) Lead poisoning. Annu Rev Med 55:209–222. https://doi.org/10.1146/annurev.med.55.091902.103653
Hon K, Fung C, Leung AK (2017) Childhood lead poisoning: an overview. Hong Kong Med J 23(6):616–621. https://doi.org/10.12809/hkmj176214
Nussbaumer-Streit B, Mayr V, Dobrescu AI, Wagner G, Chapman A, Pfadenhauer LM, Lohner S, Lhachimi SK, Busert LK, Gartlehner G (2020) Household interventions for secondary prevention of domestic lead exposure in children. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006047.pub6
Njati SY, Maguta MM (2019) Lead-based paints and children’s PVC toys are potential sources of domestic lead poisoning–a review. Environ Pollut 249:1091–1105. https://doi.org/10.1016/j.envpol.2019.03.062
Cui X-Y, Li S-W, Zhang S-J, Fan Y-Y, Ma LQ (2015) Toxic metals in children’s toys and jewelry: coupling bioaccessibility with risk assessment. Environ Pollut 200:77–84. https://doi.org/10.1016/j.envpol.2015.01.035
Romieu I, Palazuelos E, Hernandez Avila M, Rios C, Muñoz I, Jimenez C, Cahero G (1994) Sources of lead exposure in Mexico City. Environ Health Perspect 102(4):384–389. https://doi.org/10.1289/ehp.94102384
Newman N, Jones C, Page E, Ceballos D, Oza A (2015) Investigation of childhood lead poisoning from parental take-home exposure from an electronic scrap recycling facility—Ohio, 2012. MMWR Morb Mortal Wkly Rep 64(27):743
Lu X, Wang L, Lei K, Huang J, Zhai Y (2009) Contamination assessment of copper, lead, zinc, manganese and nickel in street dust of Baoji, NW China. J Hazard Mater 161(2-3):1058–1062. https://doi.org/10.1016/j.jhazmat.2008.04.052
Shabanda IS, Koki IB, Low KH, Zain SM, Khor SM, Bakar NKA (2019) Daily exposure to toxic metals through urban road dust from industrial, commercial, heavy traffic, and residential areas in Petaling Jaya, Malaysia: a health risk assessment. Environ Sci Pollut Res 26(36):37193–37211. https://doi.org/10.1007/s11356-019-06718-2
Zhang S, Dai Y, Xie X, Fan Z, Tan Z (2005) Study on blood lead level and related risk factors among children aged 0-6 years in 15 cities in China. Zhonghua Liu Xing Bing Xue Za Zhi= Zhonghua Liuxingbingxue Zazhi 26(9):651
Baranowska-Bosiacka I, Gutowska I, Rybicka M, Nowacki P, Chlubek D (2012) Neurotoxicity of lead. Hypothetical molecular mechanisms of synaptic function disorders. Neurol Neurochir Pol 46(6):569–578. https://doi.org/10.5114/ninp.2012.31607
Baranowska-Bosiacka I, Strużyńska L, Gutowska I, Machalińska A, Kolasa A, Kłos P, Czapski G, Kurzawski M, Prokopowicz A, Marchlewicz M (2013) Perinatal exposure to lead induces morphological, ultrastructural and molecular alterations in the hippocampus. Toxicology 303:187–200. https://doi.org/10.1016/j.tox.2012.10.027
Jakubowski M (2011) Low-level environmental lead exposure and intellectual impairment in children—the current concepts of risk assessment. Int J Occup Med Environ Health 24(1):1–7. https://doi.org/10.2478/s13382-011-0009-z
Hou S, Yuan L, Jin P, Ding B, Qin N, Li L, Liu X, Wu Z, Zhao G, Deng Y (2013) A clinical study of the effects of lead poisoning on the intelligence and neurobehavioral abilities of children. Theor Biol Med Model 10(1):1–9. https://doi.org/10.1186/1742-4682-10-13
Koyashiki GAK, Paoliello MMB, Tchounwou PB (2010) Lead levels in human milk and children’s health risk: a systematic review. Rev Environ Health 25(3):243–253
Rummo JH, Routh DK, Rummo NJ, Brown JF (1979) Behavioral and neurological effects of symptomatic and asymptomatic lead exposure in children. Arch Environ Health: Int J 34(2):120–124. https://doi.org/10.1080/00039896.1979.10667381
Needleman HL, Gunnoe C, Leviton A, Reed R, Peresie H, Maher C, Barrett P (1979) Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N Engl J Med 300(13):689–695. https://doi.org/10.1056/NEJM197903293001301
Needleman HL, Gatsonis CA (1990) Low-level lead exposure and the IQ of children: a meta-analysis of modern studies. Jama 263(5):673–678. https://doi.org/10.1001/jama.1990.03440050067035
Pueschel SM (1974) Neurological and psychomotor functions in children with an increased lead burden. Environ Health Perspect 7:13–16. https://doi.org/10.1289/ehp.74713
Parajuli RP, Fujiwara T, Umezaki M, Watanabe C (2013) Association of cord blood levels of lead, arsenic, and zinc with neurodevelopmental indicators in newborns: a birth cohort study in Chitwan Valley, Nepal. Environ Res 121:45–51. https://doi.org/10.1016/j.envres.2012.10.010
Gould E (2009) Childhood lead poisoning: conservative estimates of the social and economic benefits of lead hazard control. Environ Health Perspect 117(7):1162–1167. https://doi.org/10.1289/ehp.0800408
Tong S, Schirnding YEV, Prapamontol T (2000) Environmental lead exposure: a public health problem of global dimensions. Bull World Health Organ 78:1068–1077
Jusko TA, Henderson CR Jr, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL (2008) Blood lead concentrations< 10 μg/dL and child intelligence at 6 years of age. Environ Health Perspect 116(2):243–248. https://doi.org/10.1289/ehp.10424
Tong S, Baghurst P, McMichael A, Sawyer M, Mudge J (1996) Lifetime exposure to environmental lead and children’s intelligence at 11-13 years: the Port Pirie cohort study. Bmj 312(7046):1569–1575. https://doi.org/10.1136/bmj.312.7046.1569
Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T (2005) Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect 113(7):894–899. https://doi.org/10.1289/ehp.7688
Walkowiak J, Altmann L, Krämer U, Sveinsson K, Turfeld M, Weishoff-Houben M, Winneke G (1998) Cognitive and sensorimotor functions in 6-year-old children in relation to lead and mercury levels: adjustment for intelligence and contrast sensitivity in computerized testing. Neurotoxicol Teratol 20(5):511–521. https://doi.org/10.1016/S0892-0362(98)00010-5
American Academy of Pediatrics Committee on Environmental Health (2005) Lead exposure in children: prevention, detection, and management. Pediatrics 116(4):1036. https://doi.org/10.1542/peds.2005-1947
Canfield RL, Henderson CR Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP (2003) Intellectual impairment in children with blood lead concentrations below 10 μg per deciliter. N Engl J Med 348(16):1517–1526. https://doi.org/10.1056/NEJMoa022848
Binns HJ, Campbell C, Brown MJ (2007) Interpreting and managing blood lead levels of less than 10 μg/dL in children and reducing childhood exposure to lead: recommendations of the Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Pediatrics 120(5):e1285–e1298. https://doi.org/10.1542/peds.2005-1770
Advisory Committee on Childhood Lead Poisoning Prevention (2007) Interpreting and managing blood lead levels < 10 μg/dL in children and reducing childhood exposures to lead: recommendations of CDC’s Advisory Committee on Childhood Lead Poisoning Prevention. Morbidity and Mortality Weekly Report: Recommendations and Reports:1-15
Centers for Disease Control and Prevention (2012) CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in “low level lead exposure harms children: a renewed call of primary prevention”. https://www.cdc.gov/nceh/lead/resources/guidelines.html
Centers for Disease Control and Prevention (2012) What do parents need to know to protect their children. Update on Blood Lead Levels in Children. https://www.cdc.gov/nceh/lead/docs/lead-levels-in-children-fact-sheet-508.pdf
Bellinger DC (2008) Very low lead exposures and children’s neurodevelopment. Curr Opin Pediatr 20(2):172–177. https://doi.org/10.1097/MOP.0b013e3282f4f97b
Ha M, Kwon H-J, Lim M-H, Jee Y-K, Hong Y-C, Leem J-H, Sakong J, Bae J-M, Hong S-J, Roh Y-M (2009) Low blood levels of lead and mercury and symptoms of attention deficit hyperactivity in children: a report of the children’s health and environment research (CHEER). Neurotoxicology 30(1):31–36. https://doi.org/10.1016/j.neuro.2008.11.011
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM-5®), 5th ed. American Psychiatric Publishing, Washington, DC 20024
Li H, Li H, Li Y, Liu Y, Zhao Z (2018) Blood mercury, arsenic, cadmium, and lead in children with autism spectrum disorder. Biol Trace Elem Res 181(1):31–37. https://doi.org/10.1007/s12011-017-1002-6
Kanner L (1943) Autistic disturbances of affective contact. Nervous Child 2(3):217–250
Fombonne E (2003) The prevalence of autism. Jama 289(1):87–89. https://doi.org/10.1001/jama.289.1.87
Kim YS, Leventhal BL, Koh Y-J, Fombonne E, Laska E, Lim E-C, Cheon K-A, Kim S-J, Kim Y-K, Lee H (2011) Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatr 168(9):904–912. https://doi.org/10.1176/appi.ajp.2011.10101532
Baron-Cohen S, Scott FJ, Allison C, Williams J, Bolton P, Matthews FE, Brayne C (2009) Prevalence of autism-spectrum conditions: UK school-based population study. Br J Psychiatry 194(6):500–509. https://doi.org/10.1192/bjp.bp.108.059345
Blumberg SJ, Bramlett MD, Kogan MD, Schieve LA, Jones JR, Lu MC (2013) Changes in prevalence of parent-reported autism spectrum disorder in school-aged US children: 2007 to 2011-2012. vol 65. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistic
Kaur J, Engkasan J, Sivanesom R, Bahar N, Noorand M, Kamarudin K (2015) Technical report autism spectrum disorder research in Malaysia. Malaysia: Ministry of Health Malaysia. ISBN : 978-983-2387-10-7
Landrigan PJ (2010) What causes autism? Exploring the environmental contribution. Curr Opin Pediatr 22(2):219–225. https://doi.org/10.1097/MOP.0b013e328336eb9a
Grandjean P, Landrigan PJ (2006) Developmental neurotoxicity of industrial chemicals. Lancet 368(9553):2167–2178. https://doi.org/10.1016/S0140-6736(06)69665-7
Landrigan P, Baloh R, Barthel W, Whitworth R, Staehling N, Rosenblum B (1975) Neuropsychological dysfunction in children with chronic low-level lead absorption. Lancet 305(7909):708–712. https://doi.org/10.1016/S0140-6736(75)91627-X
Li Y, Hasenhuetl PS, Schicker K, Sitte HH, Freissmuth M, Sandtner W (2015) Dual action of Zn2+ on the transport cycle of the dopamine transporter. J Biol Chem 290(52):31069–31076. https://doi.org/10.1074/jbc.M115.688275
Bilgiç A, Gürkan K, Türkoğlu S, Akça ÖF, Kılıç BG, Uslu R (2010) Iron deficiency in preschool children with autistic spectrum disorders. Res Autism Spectr Disord 4(4):639–644. https://doi.org/10.1016/j.rasd.2009.12.008
Hergüner S, Keleşoğlu FM, Tanıdır C, Çöpür M (2012) Ferritin and iron levels in children with autistic disorder. Eur J Pediatr 171(1):143–146. https://doi.org/10.1007/s00431-011-1506-6
Maret W (2013) Zinc and human disease. In: Interrelations between essential metal ions and human diseases. Springer, pp 389-414
Roohani N, Hurrell R, Kelishadi R, Schulin R (2013) Zinc and its importance for human health: an integrative review. J Res Med Sci 18(2):144–157
Li Y-M, Shen Y-D, Li Y-J, Xun G-L, Liu H, Wu R-R, Xia K, Zhao J-P, Ou J-J (2018) Maternal dietary patterns, supplements intake and autism spectrum disorders: a preliminary case-control study. Medicine 97(52):e13902. https://doi.org/10.1097/MD.0000000000013902
Nanou E, Catterall WA (2018) Calcium channels, synaptic plasticity, and neuropsychiatric disease. Neuron 98(3):466–481. https://doi.org/10.1016/j.neuron.2018.03.017
Liu J, Yuan E, Zhang Z, Jia L, Yin Z, Meng X, Du H (2012) Age-and sex-specific reference intervals for blood copper, zinc, calcium, magnesium, iron, lead, and cadmium in infants and children. Clin Biochem 45(6):416–419. https://doi.org/10.1016/j.clinbiochem.2012.01.014
Swardfager W, Herrmann N, Mazereeuw G, Goldberger K, Harimoto T, Lanctôt KL (2013) Zinc in depression: a meta-analysis. Biol Psychiatry 74(12):872–878. https://doi.org/10.1016/j.biopsych.2013.05.008
Saghazadeh A, Mahmoudi M, Meysamie A, Gharedaghi M, Zamponi GW, Rezaei N (2015) Possible role of trace elements in epilepsy and febrile seizures: a meta-analysis. Nutr Rev 73(11):760–779. https://doi.org/10.1093/nutrit/nuv026
Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ (2012) GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neurosci Biobehav Rev 36(9):2044–2055. https://doi.org/10.1016/j.neubiorev.2012.07.005
Palmieri L, Papaleo V, Porcelli V, Scarcia P, Gaita L, Sacco R, Hager J, Rousseau F, Curatolo P, Manzi B (2010) Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol Psychiatry 15(1):38–52. https://doi.org/10.1038/mp.2008.63
Llinas R, Sugimori M, Silver R (1992) Microdomains of high calcium concentration in a presynaptic terminal. Science 256(5057):677–679. https://doi.org/10.1126/science.1350109
Catterall WA, Leal K, Nanou E (2013) Calcium channels and short-term synaptic plasticity. J Biol Chem 288(15):10742–10749. https://doi.org/10.1074/jbc.R112.411645
Zamponi GW, Striessnig J, Koschak A, Dolphin AC (2015) The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev 67(4):821–870. https://doi.org/10.1124/pr.114.009654
Chao H-T, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu H-C, Heintz N (2010) Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 468(7321):263–269. https://doi.org/10.1038/nature09582
Strambi M, Longini M, Hayek J, Berni S, Macucci F, Scalacci E, Vezzosi P (2006) Magnesium profile in autism. Biol Trace Elem Res 109(2):97–104. https://doi.org/10.1385/BTER:109:2:097
Johnson S (2001) Micronutrient accumulation and depletion in schizophrenia, epilepsy, autism and Parkinson’s disease? Med Hypotheses 56(5):641–645. https://doi.org/10.1054/mehy.2000.1302
Grabrucker S, Jannetti L, Eckert M, Gaub S, Chhabra R, Pfaender S, Mangus K, Reddy PP, Rankovic V, Schmeisser MJ (2014) Zinc deficiency dysregulates the synaptic ProSAP/Shank scaffold and might contribute to autism spectrum disorders. Brain 137(1):137–152. https://doi.org/10.1093/brain/awt303
Benarroch EE (2009) Brain iron homeostasis and neurodegenerative disease. Neurology 72(16):1436–1440. https://doi.org/10.1212/WNL.0b013e3181a26b30
Beard J (2003) Iron deficiency alters brain development and functioning. J Nutr 133(5):1468S–1472S. https://doi.org/10.1093/jn/133.5.1468S
Sidrak S, Yoong T, Woolfenden S (2014) Iron deficiency in children with global developmental delay and autism spectrum disorder. J Paediatr Child Health 50(5):356–361. https://doi.org/10.1111/jpc.12483
Cuadrado-Soto E, López-Sobaler AM, Jiménez-Ortega AI, Aparicio A, Bermejo LM, Hernández-Ruiz Á, Lara Villoslada F, Leis R, Martínez de Victoria E, Moreno JM (2020) Usual dietary intake, nutritional adequacy and food sources of calcium, phosphorus, magnesium and vitamin D of Spanish children aged one to< 10 years. Findings from the EsNuPI Study. Nutrients 12(6):1787. https://doi.org/10.3390/nu12061787
Ministry of Health Malaysia (2017) Recommended nutrient intakes for Malaysia 2017. A Report of the Technical Working Group on Nutritional Guidelines. National Coordinating Committee on Food and Nutrition. https://expert.taylors.edu.my/file/rems/publication/107126_3033_1.pdf. Accessed 12 January 2021
Shrimpton R, Gross R, Darnton-Hill I, Young M (2005) Zinc deficiency: what are the most appropriate interventions? Bmj 330(7487):347–349. https://doi.org/10.1136/bmj.330.7487.347
Cerami C (2017) Iron nutriture of the fetus, neonate, infant, and child. Ann Nutr Metab 71(Suppl. 3):8–14. https://doi.org/10.1159/000481447
Hashim JH, Hashim Z, Omar A, Shamsudin SB (2000) Blood lead levels of urban and rural Malaysian primary school children. Asia Pac J Public Health 12(2):65–70. https://doi.org/10.1177/101053950001200203
Shamsudin SB, Marzuki A, Jeffree MS, Lukman KA (2017) Blood lead concentration and working memory ability on Malay primary school children in urban and rural area, Malacca. Acta Scientif Malaysia 1(1):04–07. https://doi.org/10.26480/asm.01.2017.04.07
Bunnell T, Barter PA, Morshidi S (2002) Kuala Lumpur metropolitan area: a globalizing city–region. Cities 19(5):357–370. https://doi.org/10.1016/S0264-2751(02)00036-7
Ling O, Ting K, Shaharuddin A, Kadaruddin A, Yaakob M (2010) Urban growth and air quality in Kuala Lumpur city, Malaysia. The International Journal, Thai Society of Higher Education Institutes on Environment
Department of Statistics Malaysia (2020) Malaysia at a glance. Federal Territory of Kuala Lumpur. https://www.dosm.gov.my/v1/index.php?r=column/cone&menu_id=bjRlZXVGdnBueDJKY1BPWEFPRlhIdz09. Accessed 29 December 2020
Ministry of Rural Development Malaysia (2020) Official Web Portal, Community Development Division. https://www.kemas.gov.my/en/tabika-3/. Accessed 7 November 2020
Tinkov AA, Ajsuvakova OP, Skalny AV (2020) A case-control study of essential and toxic trace elements and minerals in hair of 0–4-year-old children with cerebral palsy. Biol Trace Elem Res 195(2):399–408. https://doi.org/10.1007/s12011-019-01876-3
Barbosa F Jr, Tanus-Santos JE, Gerlach RF, Parsons PJ (2005) A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Perspect 113(12):1669–1674. https://doi.org/10.1289/ehp.7917
Bergdahl IA, Skerfving S (2008) Biomonitoring of lead exposure—alternatives to blood. J Toxic Environ Health A 71(18):1235–1243. https://doi.org/10.1080/15287390802209525
Bannon DI, Portnoy ME, Olivi L, Lees PS, Culotta VC, Bressler JP (2002) Uptake of lead and iron by divalent metal transporter 1 in yeast and mammalian cells. Biochem Biophys Res Commun 295(4):978–984. https://doi.org/10.1016/S0006-291X(02)00756-8
K-s L, J-h H, Zeng Y, F-c D, P-q G (2013) Neurotoxicity and biomarkers of lead exposure: a review. Chin Med Sci J 28(3):178–188. https://doi.org/10.1016/S1001-9294(13)60045-0
Rabinowitz MB (1991) Toxicokinetics of bone lead. Environ Health Perspect 91:33–37. https://doi.org/10.1289/ehp.919133
Oflaherty EJ (1995) Physiologically based models for bone-seeking elements: V. Lead absorption and disposition in childhood. Toxicol Appl Pharmacol 131(2):297–308. https://doi.org/10.1006/taap.1995.1072
Brito JA, McNeill FE, Webber CE, Wells S, Richard N, Carvalho ML, Chettle DR (2002) Evaluation of a novel structural model to describe the endogenous release of lead from bone. J Environ Monit 4(2):194–201. https://doi.org/10.1039/B108817C
Gulson B, Mizon K, Korsch M, Howarth D, Phillips A, Hall J (1996) Impact on blood lead in children and adults following relocation from their source of exposure and contribution of skeletal tissue to blood lead. Bull Environ Contam Toxicol 56(4):543–550. https://doi.org/10.1007/s001289900078
Tsaih S-W, Schwartz J, Lee M, Amarasiriwardena C, Aro A, Sparrow D, Hu H (1999) The independent contribution of bone and erythrocyte lead to urinary lead among middle-aged and elderly men: the normative aging study. Environ Health Perspect 107(5):391–396. https://doi.org/10.1289/ehp.99107391
Gulson BL, Cameron MA, Smith AJ, Mizon KJ, Korsch MJ, Vimpani G, McMichael AJ, Pisaniello D, Jameson CW, Mahaffey KR (1998) Blood lead–urine lead relationships in adults and children. Environ Res 78(2):152–160. https://doi.org/10.1006/enrs.1997.3810
Sokas RK, Atleson J, Keogh JP (1988) Shortened forms of provocative lead chelation. J Occup Med 30(5):420–424. https://doi.org/10.1097/00043764-198805000-00008
Fukui Y, Miki M, Ukai H, Okamoto S, Takada S, Higashikawa K, Ikeda M (1999) Urinary lead as a possible surrogate of blood lead among workers occupationally exposed to lead. Int Arch Occup Environ Health 72(8):516–520. https://doi.org/10.1007/s004200050409
Gil F, Hernández A (2015) Toxicological importance of human biomonitoring of metallic and metalloid elements in different biological samples. Food Chem Toxicol 80:287–297. https://doi.org/10.1016/j.fct.2015.03.025
Gil-Hernández F, Gómez-Fernández AR, la Torre-Aguilar MJ, Pérez-Navero JL, Flores-Rojas K, Martín-Borreguero P, Gil-Campos M (2020) Neurotoxicity by mercury is not associated with autism spectrum disorders in Spanish children. Ital J Pediatr 46(1):1–7. https://doi.org/10.1186/s13052-020-0780-1
Laraque D, Trasande L (2005) Lead poisoning: successes and 21st century challenges. Pediatr Rev 26(12):429–443. https://doi.org/10.1542/pir.26-12-435
Min J-Y, Min K-B, Cho S-I, Kim R, Sakong J, Paek D (2007) Neurobehavioral function in children with low blood lead concentrations. Neurotoxicology 28(2):421–425. https://doi.org/10.1016/j.neuro.2006.03.007
Chiodo LM, Jacobson SW, Jacobson JL (2004) Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol Teratol 26(3):359–371. https://doi.org/10.1016/j.ntt.2004.01.010
Surkan PJ, Zhang A, Trachtenberg F, Daniel DB, McKinlay S, Bellinger DC (2007) Neuropsychological function in children with blood lead levels< 10 μg/dL. Neurotoxicology 28(6):1170–1177. https://doi.org/10.1016/j.neuro.2007.07.007
Emory E, Ansari Z, Pattillo R, Archibold E, Chevalier J (2003) Maternal blood lead effects on infant intelligence at age 7 months. Am J Obstet Gynecol 188(4):S26–S32. https://doi.org/10.1067/mob.2003.244
Jedrychowski W, Perera F, Jankowski J, Rauh V, Flak E, Caldwell KL, Jones RL, Pac A, Lisowska-Miszczyk I (2008) Prenatal low-level lead exposure and developmental delay of infants at age 6 months (Krakow inner city study). Int J Hyg Environ Health 211(3-4):345–351. https://doi.org/10.1016/j.ijheh.2007.07.023
Lucchini RG, Zoni S, Guazzetti S, Bontempi E, Micheletti S, Broberg K, Parrinello G, Smith DR (2012) Inverse association of intellectual function with very low blood lead but not with manganese exposure in Italian adolescents. Environ Res 118:65–71. https://doi.org/10.1016/j.envres.2012.08.003
Koller K, Brown T, Spurgeon A, Levy L (2004) Recent developments in low-level lead exposure and intellectual impairment in children. Environ Health Perspect 112(9):987–994. https://doi.org/10.1289/ehp.6941
Elias S, Hashim Z, Marjan Z, Abdullah A, Hashim J (2007) Relationship between blood lead concentration and nutritional status among Malay primary school children in Kuala Lumpur, Malaysia. Asia Pac J Public Health 19(3):29–37. https://doi.org/10.1177/101053950701900306
Lovei M (1998) Phasing out lead from gasoline: worldwide experience and policy implications. The World Bank Technical Paper No. 397. Pollution Management Series. Washington D.C.
Hirota K (2010) Comparative studies on vehicle related policies for air pollution reduction in ten Asian countries. Sustainability 2(1):145–162. https://doi.org/10.3390/su2010145
Ministry of International Trade and Industry Malaysia (2020) National Automotive Policy (NAP) 2020. https://www.miti.gov.my/index.php/pages/view/nap2020. Accessed on 7 Jan 2021
Food Packaging Forum Foundation (2020) Malaysia sets metal migration limits for ceramics-news. https://www.foodpackagingforum.org/news/malaysia-sets-metal-migration-limits-for-ceramics. Accessed 8 January 2021
Ministry of Health Malaysia (2017) Guidelines on importation of ceramic ware intended to be used in the preparation, packaging, storage, delivery or exposure of food for human consumption. Food Safety & Quality Division. http://fsq.moh.gov.my/v6/xs/dl.php?filename=4ec4c9f481b23b62ec9680d4a0beb5ed.pdf. Accessed 8 January 2021
United Nations Environment Programme (2015) INF/25 Status of the phasing out of lead paint by countries: 2015. Global report, at the International Conference on Chemicals Management Fourth session, Geneva, 2015. http://www.saicm.org/Portals/12/documents/meetings/ICCM4/inf/ICCM4_INF25_Lead_in_Paint_2015.doc. Accessed 8 January 2021
Yong YS, Lim YA, Ilankoon I (2019) An analysis of electronic waste management strategies and recycling operations in Malaysia: challenges and future prospects. J Clean Prod 224:151–166. https://doi.org/10.1016/j.jclepro.2019.03.205
Shearer T, Larson K, Neuschwander J, Gedney B (1982) Minerals in the hair and nutrient intake of autistic children. J Autism Dev Disord 12(1):25–34. https://doi.org/10.1007/BF01531671
Marlowe M, Errera J (1985) Hair mineral content as a predictor of childhood autism. Monograph in Behavioral Disorder US Department of Education, National Institute of Education, Educational Resources Information Center (ERIC):113
Yasuda H, Yonashiro T, Yoshida K, Ishii T, Tsutsui T (2005) Mineral imbalance in children with autistic disorders. Biomed Res Trace Elem 16(4):285–292. https://doi.org/10.11299/brte.16.285
Adams J, Holloway C, George F, Quig D (2006) Analyses of toxic metals and essential minerals in the hair of Arizona children with autism and associated conditions, and their mothers. Biol Trace Elem Res 110(3):193–209. https://doi.org/10.1385/BTER:110:3:193
Kern JK, Grannemann BD, Trivedi MH, Adams JB (2007) Sulfhydryl-reactive metals in autism. J Toxic Environ Health A 70(8):715–721. https://doi.org/10.1080/15287390601188060
Yorbik Ö, Kurt İ, Haşimi A, Öztürk Ö (2010) Chromium, cadmium, and lead levels in urine of children with autism and typically developing controls. Biol Trace Elem Res 135(1-3):10–15. https://doi.org/10.1007/s12011-009-8494-7
Obrenovich ME, Shamberger RJ, Lonsdale D (2011) Altered heavy metals and transketolase found in autistic spectrum disorder. Biol Trace Elem Res 144(1-3):475–486. https://doi.org/10.1007/s12011-011-9146-2
Abdullah MM, Ly AR, Goldberg WA, Clarke-Stewart KA, Dudgeon JV, Mull CG, Chan TJ, Kent EE, Mason AZ, Ericson JE (2012) Heavy metal in children’s tooth enamel: related to autism and disruptive behaviors? J Autism Dev Disord 42(6):929–936. https://doi.org/10.1007/s10803-011-1318-6
Albizzati A, More L, Di Candia D, Saccani M, Lenti C (2012) Normal concentrations of heavy metals in autistic spectrum disorders. Minerva Pediatr 64(1):27–31
Alabdali A, Al-Ayadhi L, El-Ansary A (2014) A key role for an impaired detoxification mechanism in the etiology and severity of autism spectrum disorders. Behav Brain Funct 10(1):14. https://doi.org/10.1186/1744-9081-10-14
Rahbar MH, Samms-Vaughan M, Dickerson AS, Loveland KA, Ardjomand-Hessabi M, Bressler J, Shakespeare-Pellington S, Grove ML, Pearson DA, Boerwinkle E (2015) Blood lead concentrations in Jamaican children with and without autism spectrum disorder. Int J Environ Res Public Health 12(1):83–105. https://doi.org/10.3390/ijerph120100083
Fuentes-Albero M, Puig-Alcaraz C, Cauli O (2015) Lead excretion in Spanish children with autism spectrum disorder. Brain Sci 5(1):58–68. https://doi.org/10.3390/brainsci5010058
Skalny AV, Simashkova NV, Klyushnik TP, Grabeklis AR, Bjørklund G, Skalnaya MG, Nikonorov AA, Tinkov AA (2017) Hair toxic and essential trace elements in children with autism spectrum disorder. Metab Brain Dis 32(1):195–202. https://doi.org/10.1007/s11011-016-9899-6
Gaza M, Hakim L, Sabarudin A, Sumitro S (2017) Evaluation on mercury, cadmium, and lead in the hair sample as an indicator of autism for children. Int J Pharm Clin Res 9(12):710–715 ISSN- 0975 1556
Rahbar MH, Samms-Vaughan M, Lee M, Zhang J, Hessabi M, Bressler J, Bach MA, Grove ML, Shakespeare-Pellington S, Beecher C (2020) Interaction between a mixture of heavy metals (lead, mercury, arsenic, cadmium, manganese, aluminum) and GSTP1, GSTT1, and GSTM1 in relation to autism spectrum disorder. Res Autism Spectr Disord 79:101681. https://doi.org/10.1016/j.rasd.2020.101681
Bradstreet J, Geier DA, Kartzinel JJ, Adams JB, Geier MR (2003) A case-control study of mercury burden in children with autistic spectrum disorders. J Am Phys Surg 8(3):76–79
Holmes AS, Blaxill MF, Haley BE (2003) Reduced levels of mercury in first baby haircuts of autistic children. Int J Toxicol 22(4):277–285. https://doi.org/10.1080/10915810305120
Bjørklund G (2013) The role of zinc and copper in autism spectrum disorders. Acta Neurobiol Exp (Wars) 73(2):225–236
Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, Dalla Bernardina B, Bonassi S (2012) Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic Biol Med 52(10):2128–2141. https://doi.org/10.1016/j.freeradbiomed.2012.03.011
Bjørklund G, Tinkov AA, Hosnedlová B, Kizek R, Ajsuvakova OP, Chirumbolo S, Skalnaya MG, Peana M, Dadar M, El-Ansary A (2020) The role of glutathione redox imbalance in autism spectrum disorder: a review. Free Radic Biol Med 160:149–162. https://doi.org/10.1016/j.freeradbiomed.2020.07.017
Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QM (2015) Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci 16(12):29592–29630. https://doi.org/10.3390/ijms161226183
Skalny AV, Mazaletskaya AL, Ajsuvakova OP, Bjørklund G, Skalnaya MG, Chernova LN, Skalny AA, Tinkov AA (2020) Magnesium status in children with attention-deficit/hyperactivity disorder and/or autism spectrum disorder. J Korean Acad Child Adolesc Psychiatry 31(1):41–45. https://doi.org/10.5765/jkacap.190036
Priya MDL, Geetha A (2011) Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol Trace Elem Res 142(2):148–158. https://doi.org/10.1007/s12011-010-8766-2
Saghazadeh A, Ahangari N, Hendi K, Saleh F, Rezaei N (2017) Status of essential elements in autism spectrum disorder: systematic review and meta-analysis. Rev Neurosci 28(7):783–809. https://doi.org/10.1515/revneuro-2017-0015
Li S-o, Wang J-l, Bjørklund G, Zhao W-N, Yin C-H (2014) Serum copper and zinc levels in individuals with autism spectrum disorders. Neuroreport 25(15):1216–1220. https://doi.org/10.1097/WNR.0000000000000251
Lubkowska A, Sobieraj W (2009) Concentrations of magnesium, calcium, iron, selenium, zinc and copper in the hair of autistic children. Trace Elements Electrolytes 26(2):72–77. https://doi.org/10.5414/TEP26072
Tinkov AA, Skalnaya MG, Simashkova NV, Klyushnik TP, Skalnaya AA, Bjørklund G, Notova SV, Kiyaeva EV, Skalny AV (2019) Association between catatonia and levels of hair and serum trace elements and minerals in autism spectrum disorder. Biomed Pharmacother 109:174–180. https://doi.org/10.1016/j.biopha.2018.10.051
Eow SY, Gan WY, Lim PY, Awang H, Shariff ZM (2020) Factors associated with autism severity among Malaysian children with Autism Spectrum Disorder. Res Dev Disabil 100:103632. https://doi.org/10.1016/j.ridd.2020.103632
Fombonne E (2009) Epidemiology of pervasive developmental disorders. Pediatr Res 65(6):591–598
Baxter AJ, Brugha T, Erskine HE, Scheurer RW, Vos T, Scott JG (2015) The epidemiology and global burden of autism spectrum disorders. Psychol Med 45(3):601–613. https://doi.org/10.1017/S003329171400172X
Loomes R, Hull L, Mandy WPL (2017) What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 56(6):466–474. https://doi.org/10.1016/j.jaac.2017.03.013
Zhang X, Lv C-C, Tian J, Miao R-J, Xi W, Hertz-Picciotto I, Qi L (2010) Prenatal and perinatal risk factors for autism in China. J Autism Dev Disord 40(11):1311–1321. https://doi.org/10.1007/s10803-010-0992-0
Acknowledgements
We would like to express appreciation to the Department of Community Health and Faculty of Medicine, the National University of Malaysia (UKM) for the assistance given for this research. We also would like to thank the Genius Kurnia, Centre of Excellence of Early Intervention for Children with Autism, Ministry of Education (MOE) Malaysia and KEMAS, Ministry of Rural Development (MRD) Malaysia for the permission given to conduct the study.
Funding
This study was funded by UKM research and ethics committee: FF-2018-286.
Author information
Authors and Affiliations
Contributions
This article is a requirement of SW’s Doctor of Philosophy (Ph.D.) under the supervision of HJ and ZI. All authors designed the research project. The ethical approval was obtained by SW and HJ. SW conducted the research, collected the data, performed the laboratory analysis, analysed the data and interpreted the findings. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This study was approved by the UKM research and ethics committee: FF-2018-286 and the NMRR from MOH Malaysia: NMRR-18-3765-45117.
Consent to Participate
Written informed consent was obtained from the parents.
Consent for Publication
Patients signed informed consent regarding publishing their data.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd Wahil, M.S., Ja’afar, M.H. & Md Isa, Z. Assessment of Urinary Lead (Pb) and Essential Trace Elements in Autism Spectrum Disorder: a Case-Control Study Among Preschool Children in Malaysia. Biol Trace Elem Res 200, 97–121 (2022). https://doi.org/10.1007/s12011-021-02654-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02654-w