Abstract
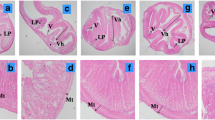
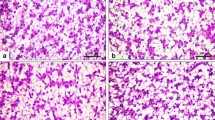
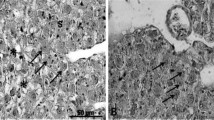
Soybean meal (SBM) has been commonly utilised as a substitute for fishmeal (FM) in the diets of several fish species. However, little is known regarding their effects on trace element availability and thus their importance to fish. The present study employed two feeding trials to evaluate the implications of dietary selenium (Se) on the growth, accumulation, antioxidant, and histopathological responses of juvenile barramundi (Lates calcarifer). In the first trial, each of three basal diets containing 0, 15 and 43 % SBM as replacements for 0, 25 and 75 % of FM protein on an isoproteic and isocalorific basis were either supplemented or not supplemented with 2 mg kg−1 organic Se (OS). In the second trial, the potential effect of OS supplementation in a high SBM diet was investigated in a feeding trial with five experimental diets: 75 % SBM protein as replacement of FM was supplemented with 2, 3, 4, 5 or 7 mg OS kg−1. Growth was independently influenced by the SBM level and the OS supplementation level but not by their interaction. Glutathione peroxidase (GPx) activity, haematocrit, Se accumulation and muscle tissue integrity were significantly enhanced in fish fed on OS-supplemented diets. Furthermore, when high SBM was included in diets, elevated Se tended to lower the barramundi’s performance. These findings suggest that dietary supplementation of OS at 2–3 g kg−1 diet is necessary when high plant protein ingredients are incorporated in the diet, in order to maintain better growth and to afford protection against oxidative stress.


Similar content being viewed by others
References
Sarker PK, Bureau DP, Hua K, Drew MD, Forster I, Were K, Hicks B, Vandenberg GW (2013) Sustainability issues related to feeding salmonids: a Canadian perspective. Rev Aquac 5(4):199–219. doi:10.1111/raq.12013
Troell M, Kautsky N, Beveridge M, Henriksson P, Primavera J, Rönnbäck P, Folke C (2013) Aquaculture. In: Levin SA (ed) Encyclopedia of biodiversity, Second edn. Academic Press, Waltham, pp. 189–201. doi:10.1016/B978-0-12-384719-5.00307-5
Globefish (2015) Fishmeal and fish oil - august 2015. FAO. http://www.globefish.org/fishmeal-and-fish-oil-aug-2015.html. Accessed 10 November 2015
Villasante S, Rodríguez-González D, Antelo M, Rivero-Rodríguez S, Lebrancón-Nieto J (2013) Why are prices in wild catch and aquaculture industries so different? Ambio 42(8):937–950. doi:10.1007/s13280-013-0449-8
Tacon AGJ, Metian M (2009) Fishing for aquaculture: non-food use of small pelagic forage fish—a global perspective. Rev Fish Sci 17(3):305–317. doi:10.1080/10641260802677074
Tacon AGJ, Metian M (2015) Feed matters: satisfying the feed demand of aquaculture. Rev Fish Sci Aquac 23(1):1–10. doi:10.1080/23308249.2014.987209
Krogdahl Å, Penn M, Thorsen J, Refstie S, Bakke AM (2010) Important antinutrients in plant feedstuffs for aquaculture: an update on recent findings regarding responses in salmonids. Aquac Res 41(3):333–344. doi:10.1111/j.1365-2109.2009.02426.x
Kumar V, Sinha AK, Makkar HPS, De Boeck G, Becker K (2012) Phytate and phytase in fish nutrition. J Anim Physiol Anim Nutr (Berl) 96(3):335–364. doi:10.1111/j.1439-0396.2011.01169.x
Harland BF, Morris ER (1995) Phytate: a good or a bad food component? Nutr Res 15(5):733–754. doi:10.1016/0271-5317(95)00040-P
Connelly P (2011) Nutritional advantages and disadvantages of dietary phytates: part 1. J Aust Tradit-Med Soc 17(1):21–24
Sathe SK, Reddy NR (2002) Introduction. In: Reddy NR, Sathe SK (eds) Food Phytates. CRC Press, Florida, USA, p. 280
Storebakken T, Shearer KD, Roem AJ (1998) Availability of protein, phosphorus and other elements in fish meal, soy-protein concentrate and phytase-treated soy-protein-concentrate-based diets to Atlantic salmon, Salmo salar. Aquaculture 161(1–4):365–379. doi:10.1016/S0044-8486(97)00284-6
Usmani N, Jafri AK (2002) Influence of dietary phytic acid on the growth, conversion efficiency, and carcass composition of mrigal Cirrhinus mrigala (Hamilton) fry. J World Aquacult Soc 33(2):199–204. doi:10.1111/j.1749-7345.2002.tb00495.x
Ashmead HD (1992) The roles of amino acids chelates in animal nutrition. Noyes Publications, Park Ridge, New Jersey
Apines-Amar MJS, Satoh S, Caipang CMA, Kiron V, Watanabe T, Aoki T (2004) Amino acid-chelate: a better source of Zn, Mn and Cu for rainbow trout, Oncorhynchus mykiss. Aquaculture 240(1–4):345–358. doi:10.1016/j.aquaculture.2004.01.032
Cotter P (2006) Dietary selenium in cultured hybrid striped bass. Virginia Polytechnic Institute and State University, Virginia
Fontagne-Dicharry S, Godin S, Liu H, Antony Jesu Prabhu P, Bouyssiere B, Bueno M, Tacon P, Medale F, Kaushik SJ (2015) Influence of the forms and levels of dietary selenium on antioxidant status and oxidative stress-related parameters in rainbow trout (Oncorhynchus mykiss) fry. Br J Nutr 113(12):1876–1887. doi:10.1017/s0007114515001300
Le KT, Fotedar R (2014) Bioavailability of selenium from different dietary sources in yellowtail kingfish (Seriola lalandi). Aquaculture 420–421 (0):57–62. doi:10.1016/j.aquaculture.2013.10.034
Gatlin DM, Poe WE, Wilson RP (1986) Effects of singular and combined dietary deficiencies of selenium and vitamin E on fingerling channel catfish (Ictalurus punctatus). J Nutr 116(6):1061–1067
Watanabe T, Kiron V, Satoh S (1997) Trace minerals in fish nutrition. Aquaculture 151(1–4):185–207. doi:10.1016/s0044-8486(96)01503-7
Jaramillo F Jr, Peng LI, Gatlin Iii DM (2009) Selenium nutrition of hybrid striped bass (Morone chrysops × M. saxatilis) bioavailability, toxicity and interaction with vitamin E. Aquac Nutr 15(2):160–165. doi:10.1111/j.1365-2095.2008.00579.x
Lall SP (2003) 5 - the minerals. In: John EH, Ronald WH (eds) Fish nutrition, Third edn. Academic Press, San Diego, pp. 259–308
Liu K, Wang XJ, Ai Q, Mai K, Zhang W (2010) Dietary selenium requirement for juvenile cobia, Rachycentron canadum L. Aquac Res 41(10):e594–e601. doi:10.1111/j.1365-2109.2010.02562.x
Ashouri S, Keyvanshokooh S, Salati AP, Johari SA, Pasha-Zanoosi H (2015) Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture 446:25–29. doi:10.1016/j.aquaculture.2015.04.021
Le KT, Fotedar R (2013) Dietary selenium requirement of yellowtail kingfish (Seriola lalandi). Agric Sci 4:68–75. doi:10.4236/as.2013.46A011
Lin Y-H, Shiau S-Y (2005) Dietary selenium requirements of juvenile grouper, Epinephelus malabaricus. Aquaculture 250(1–2):356–363. doi:10.1016/j.aquaculture.2005.03.022
Kucukbay FZ, Yazlak H, Karaca I, Sahin N, Tuzcu M, Cakmak MN, Sahin K (2009) The effects of dietary organic or inorganic selenium in rainbow trout (Oncorhynchus mykiss) under crowding conditions. Aquac Nutr 15(6):569–576. doi:10.1111/j.1365-2095.2008.00624.x
Arshad U, Takami GA, Sadeghi M, Bai S, Pourali HR, Lee S (2011) Influence of dietary l-selenomethionine exposure on growth and survival of juvenile Huso huso. J Appl Ichthyol 27(2):761–765. doi:10.1111/j.1439-0426.2010.01639.x
Han D, Xie S, Liu M, Xiao X, Liu H, Zhu X, Yang Y (2011) The effects of dietary selenium on growth performances, oxidative stress and tissue selenium concentration of gibel carp (Carassius auratus gibelio). Aquac Nutr 17(3):e741–e749. doi:10.1111/j.1365-2095.2010.00841.x
Wang KY, Peng CZ, Huang JL, Huang YD, Jin MC, Geng Y (2013) The pathology of selenium deficiency in Cyprinus carpio L. J Fish Dis 36(7):609–615. doi:10.1111/jfd.12030
Le KT, Fotedar R (2014) Immune responses to Vibrio anguillarum in yellowtail kingfish, Seriola lalandi, fed selenium supplementation. J World Aquacult Soc 45(2):138–148. doi:10.1111/jwas.12104
Paul H, Chris C, Marty P (2013) Farming of barramundi/Asian Seabass. In: Biology and Culture of Asian Seabass Lates calcarifer. CRC Press, pp 259–272. doi:10.1201/b15974-11
Glencross B, Blyth D, Irvin S, Bourne N, Campet M, Boisot P, Wade NM (2016) An evaluation of the complete replacement of both fishmeal and fish oil in diets for juvenile Asian seabass, Lates calcarifer. Aquaculture 451:298–309. doi:10.1016/j.aquaculture.2015.09.012
Glencross BD, Rutherford N, Jones B (2011) Evaluating options for fishmeal replacement in diets for juvenile barramundi (Lates calcarifer). Aquac Nutr 17(3):e722–e732. doi:10.1111/j.1365-2095.2010.00834.x
Katersky RS, Carter CG (2009) Growth and protein synthesis of barramundi, Lates calcarifer, fed lupin as a partial protein replacement. Comp Biochem Physiol A Mol Integr Physiol 152(4):513–517. doi:10.1016/j.cbpa.2008.12.017
Ngo DT, Pirozzi I, Glencross B (2015) Digestibility of canola meals in barramundi (Asian seabass; Lates calcarifer). Aquaculture 435 (0):442–449. doi:10.1016/j.aquaculture.2014.10.031
Tabrett S, Blyth D, Bourne N, Glencross BD (2012) Digestibility of Lupinus albus lupin meals in barramundi (Lates calcarifer). Aquaculture 364-365:1–5. doi:10.1016/j.aquaculture.2012.07.024
Van Vo B, Bui DP, Nguyen HQ, Fotedar R (2015) Optimized fermented lupin (Lupinus angustifolius) inclusion in juvenile barramundi (Lates calcarifer) diets. Aquaculture 444:62–69. doi:10.1016/j.aquaculture.2015.03.019
NRC (2011) Nutrient requirements of fish and shrimp. National Academies Press, Washington, D.C.
McLeay DJ, Gordon MR (1977) Leucocrit: a simple hematological technique for measuring acute stress in salmonid fish, including stressful concentrations of pulpmill effluent. J Fish Res Board Can 34(11):2156–2163. doi:10.1139/f77-284
AOAC (1990) Official methods of analysis of the Association of Official Analytical Chemists, vol 1, 15 edn. The Association of Official Analytical Chemists, Arlington
Barkholt V, Jensen AL (1989) Amino acid analysis: determination of cysteine plus half-cystine in proteins after hydrochloric acid hydrolysis with a disulfide compound as additive. Anal Biochem 177(2):318–322
Rayner CJ (1985) Protein hydrolysis of animal feeds for amino acid content. J Agric Food Chem 33(4):722–725. doi:10.1021/jf00064a039
Gratzfeld-Huesgen A (1998) Sensitive and reliable amino acid analysis in protein hydrolysates using the Agilent 1100 Series HPLC. Agilent Technologies Publication Number 5968-5658E
Dulski TR (1996) A manual for the chemical analysis for metals. American society for testing and materials. West Conshohocken, PA
Luna LG (ed) (1968) Manual of histological staining methods of the armed forces Institute of Pathology, 3rd edn. New York, McGraw-Hill Book Company
Chou RL, Her BY, Su MS, Hwang G, Wu YH, Chen HY (2004) Substituting fish meal with soybean meal in diets of juvenile cobia Rachycentron canadum. Aquaculture 229(1–4):325–333. doi:10.1016/S0044-8486(03)00395-8
Zhou QC, Mai KS, Tan BP, Liu YJ (2005) Partial replacement of fishmeal by soybean meal in diets for juvenile cobia (Rachycentron canadum). Aquac Nutr 11(3):175–182. doi:10.1111/j.1365-2095.2005.00335.x
Wang Y, Kong L-J, Li C, Bureau DP (2006) Effect of replacing fish meal with soybean meal on growth, feed utilization and carcass composition of cuneate drum (Nibea miichthioides). Aquaculture 261(4):1307–1313. doi:10.1016/j.aquaculture.2006.08.045
Nengas I, Alexis MN, Davies SJ (1996) Partial substitution of fishmeal with soybean meal products and derivatives in diets for the gilthead sea bream Sparus aurata (L.). Aquac Res 27(3):147–156. doi:10.1111/j.1365-2109.1996.tb00979.x
Bowyer JN, Qin JG, Smullen RP, Adams LR, Thomson MJS, Stone DAJ (2013) The use of a soy product in juvenile yellowtail kingfish (Seriola lalandi) feeds at different water temperatures: 1. Solvent extracted soybean meal. Aquaculture 384–387 (0):35–45. doi:10.1016/j.aquaculture.2012.12.005
Barrows FT, Stone DAJ, Hardy RW (2007) The effects of extrusion conditions on the nutritional value of soybean meal for rainbow trout (Oncorhynchus mykiss). Aquaculture 265(1–4):244–252. doi:10.1016/j.aquaculture.2007.01.017
Bonaldo A, Roem AJ, Pecchini A, Grilli E, Gatta PP (2006) Influence of dietary soybean meal levels on growth, feed utilization and gut histology of Egyptian sole (Solea aegyptiaca) juveniles. Aquaculture 261(2):580–586. doi:10.1016/j.aquaculture.2006.08.013
Kader MA, Bulbul M, Koshio S, Ishikawa M, Yokoyama S, Nguyen BT, Komilus CF (2012) Effect of complete replacement of fishmeal by dehulled soybean meal with crude attractants supplementation in diets for red sea bream, Pagrus major. Aquaculture 350-353:109–116. doi:10.1016/j.aquaculture.2012.04.009
Arndt RE, Hardy RW, Sugiura SH, Dong FM (1999) Effects of heat treatment and substitution level on palatability and nutritional value of soy defatted flour in feeds for Coho Salmon, Oncorhynchus kisutch. Aquaculture 180(1–2):129–145. doi:10.1016/S0044-8486(99)00186-6
Kissil GW, Lupatsch I, Higgs DA, Hardy RW (2000) Dietary substitution of soy and rapeseed protein concentrates for fish meal, and their effects on growth and nutrient utilization in gilthead seabream Sparus aurata L. Aquac Res 31(7):595–601. doi:10.1046/j.1365-2109.2000.00477.x
Richardson NL, Higgs DA, Beames RM, McBride JR (1985) Influence of dietary calcium, phosphorus, zinc and sodium phytate level on cataract incidence, growth and histopathology in juvenile chinook salmon (Oncorhynchus tshawytscha). J Nutr 115(5):553–567
Wang Y-B, Xu B-H (2008) Effect of different selenium source (sodium selenite and selenium yeast) on broiler chickens. Anim Feed Sci Tech 144 (3–4):306–314. doi:10.1016/j.anifeedsci.2007.10.012
Zhou X, Wang Y, Gu Q, Li W (2009) Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture 291(1–2):78–81. doi:10.1016/j.aquaculture.2009.03.007
Nugroho RA, Fotedar R (2013) Dietary organic selenium improves growth, survival and resistance to Vibrio mimicus in cultured marron, Cherax cainii (Austin, 2002). Fish Shellfish Immunol 35(1):79–85. doi:10.1016/j.fsi.2013.04.011
Penglase S, Nordgreen A, van der Meeren T, Olsvik PA, Sæle Ø, Sweetman JW, Baeverfjord G, Helland S, Hamre K (2010) Increasing the level of selenium in rotifers (Brachionus plicatilis ‘Cayman’) enhances the mRNA expression and activity of glutathione peroxidase in cod (Gadus morhua L.) larvae. Aquaculture 306(1–4):259–269. doi:10.1016/j.aquaculture.2010.05.011
Wang C, Lovell RT (1997) Organic selenium sources, selenomethionine and selenoyeast, have higher bioavailability than an inorganic selenium source, sodium selenite, in diets for channel catfish (Ictalurus punctatus). Aquaculture 152(1–4):223–234. doi:10.1016/s0044-8486(96)01523-2
Adams SM, Brown AM, Goede RW (1993) A quantitative health assessment index for rapid rvaluation of fish condition in the field. Trans Am Fis Soc 122(1):63–73. doi:10.1577/1548-8659(1993)122<0063:AQHAIF>2.3.CO;2
Anderson IG, Schaumuller LF, Kramer HL (1996) A preliminary study on the hematology of freshwater-reared sea bass/barramundi, Lates calcarifer. Asian Fish Sci 9:101–107
Le KT, Dao TTT, Fotedar R, Partrigde GJ (2014) Effects of variation in dietary contents of selenium and vitamin E on growth and physiological and haematological responses of yellowtail kingfish, Seriola lalandi. Aquac Int 22(2):435–446. doi:10.1007/s10499-013-9651-8
Abdel-Tawwab M, Mousa MAA, Abbass FE (2007) Growth performance and physiological response of African catfish, Clarias gariepinus (B.) fed organic selenium prior to the exposure to environmental copper toxicity. Aquaculture 272(1–4):335–345. doi:10.1016/j.aquaculture.2007.09.004
Hao X, Ling Q, Hong F (2014) Effects of dietary selenium on the pathological changes and oxidative stress in loach (Paramisgurnus dabryanus). Fish Physiol Biochem 40(5):1313–1323. doi:10.1007/s10695-014-9926-7
Kim J-H, Kang J-C (2014) The selenium accumulation and its effect on growth, and haematological parameters in red sea bream, Pagrus major, exposed to waterborne selenium. Ecotoxicol Environ Saf 104:96–102. doi:10.1016/j.ecoenv.2014.02.010
Wedemeyer GA (1996) Basic physiological functions. Physiology of Fish in Intensive Culture Systems. Springer US, In, pp. 10–59. doi:10.1007/978-1-4615-6011-1_2
Hoffmann PR, Berry MJ (2008) The influence of selenium on immune responses. Mol Nutr Food Res 52(11):1273–1280. doi:10.1002/mnfr.200700330
Köhrle J, Brigelius-Flohé R, Böck A, Gärtner R, Meyer O, Flohé L (2000) Selenium in biology: facts and medical perspectives. Biol Chem 381(9–10):849–864. doi:10.1515/bc.2000.107
Neve J, Richard M-J (1994) Concenpts and effects of intervention studies with Antioxidative trace elements in humans. In: Favier AE, Neve J, Faure P (eds) Trace elements and free radicals in oxidative diseases. The American Oil Chemists Society Press, Champaign, Illinois, p. 310
Brown KM, Arthur JR (2001) Selenium, selenoproteins and human health: a review. Public Health Nutr 4(2B):593–599. doi:10.1079/PHN2001143
Yamashita Y, Yabu T, Yamashita M (2010) Discovery of the strong antioxidant selenoneine in tuna and selenium redox metabolism. World J Biol Chem 1(5):144–150. doi:10.4331/wjbc.v1.i5.144
Elia AC, Prearo M, Pacini N, Dörr AJM, Abete MC (2011) Effects of selenium diets on growth, accumulation and antioxidant response in juvenile carp. Ecotoxicol Environ Saf 74(2):166–173. doi:10.1016/j.ecoenv.2010.04.006
Gatlin DM, Wilson RP (1984) Dietary selenium requirement of fingerling channel catfish. J Nutr 114 (3):627–633. doi:None
Bell JG, Cowey CB, Adron JW, Pirie BJS (1987) Some effects of selenium deficiency on enzyme activities and indices of tissue peroxidation in Atlantic salmon parr (Salmo salar). Aquaculture 65(1):43–54. doi:10.1016/0044-8486(87)90269-9
Shan Z, Li H, Bao X, He C, Yu H, Liu W, Hou L, Wang J, Zhu D, Sui L, Zhu B, Li Y (2011) A selenium-dependent glutathione peroxidase in the Japanese scallop, Mizuhopecten yessoensis: cDNA cloning, promoter sequence analysis and mRNA expression. Comp Biochem Physiol B Biochem Mol Biol 159(1):1–9. doi:10.1016/j.cbpb.2011.01.003
Burk RF, Hill KE (1993) Regulation of selenoproteins. Annu Rev Nutr 13:65–81. doi:10.1146/annurev.nu.13.070193.000433
Prabhu PAJ, Schrama JW, Kaushik SJ (2014) Mineral requirements of fish: a systematic review. Rev Aquac 6:1–48. doi:10.1111/raq.12090
Hilton JW, Hodson PV, Slinger SJ (1980) The requirement and toxicity of selenium in rainbow trout (Salmo gairdneri). J Nutr 110 (12):2527–2535. doi:None
Misra S, Peak D, Chen N, Hamilton C, Niyogi S (2012) Tissue-specific accumulation and speciation of selenium in rainbow trout (Oncorhynchus mykiss) exposed to elevated dietary selenomethionine. Comp Biochem Physiol C Toxicol Pharmacol 155(4):560–565. doi:10.1016/j.cbpc.2012.01.005
Lorentzen M, Maage A, Julshamn K (1994) Effects of dietary selenite or selenomethionine on tissue selenium levels of Atlantic salmon (Salmo salar). Aquaculture 121(4):359–367. doi:10.1016/0044-8486(94)90270-4
McCord JM (2000) The evolution of free radicals and oxidative stress. Am J Med 108(8):652–659. doi:10.1016/S0002-9343(00)00412-5
Atencio L, Moreno I, Jos Á, Prieto AI, Moyano R, Blanco A, Cameán AM (2009) Effects of dietary selenium on the oxidative stress and pathological changes in tilapia (Oreochromis niloticus) exposed to a microcystin-producing cyanobacterial water bloom. Toxicon 53(2):269–282. doi:10.1016/j.toxicon.2008.11.011
Tashjian DH, Teh SJ, Sogomonyan A, Hung SSO (2006) Bioaccumulation and chronic toxicity of dietary l-selenomethionine in juvenile white sturgeon (Acipenser transmontanus). Aquat Toxicol 79(4):401–409. doi:10.1016/j.aquatox.2006.07.008
Hamilton SJ, Buhl KJ, Faerber NL, Bullard FA, Wiedmeyer RH (1990) Toxicity of organic selenium in the diet to chinook salmon. Environ Toxicol Chem 9(3):347–358. doi:10.1002/etc.5620090310
Acknowledgments
This research was financially sponsored by the Australia Awards Scholarship and Department of Environment and Agriculture of Curtin University. The authors would like to thank the Animal Health Laboratories of the Department of Food and Agriculture Western Australia for laboratory assistance. We also wish to thank Dr. Ky Trung Le for his help during the sampling at the end of the research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
Fish-handling procedures, care and facilities complied with the guidelines of the Animal Ethics Committee of Curtin University (Approval Number: AEC-2013-07) and followed the Australian Code of Practise for the care and use of animals for scientific purposes.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ilham, I., Siddik, M.A.B. & Fotedar, R. Effects of Organic Selenium Supplementation on Growth, Accumulation, Haematology and Histopathology of Juvenile Barramundi (Lates calcarifer) Fed High Soybean Meal Diets. Biol Trace Elem Res 174, 436–447 (2016). https://doi.org/10.1007/s12011-016-0708-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0708-1