Abstract
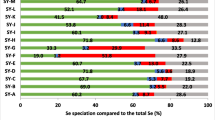
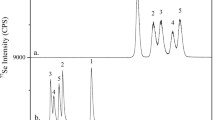
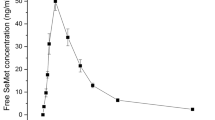
The trace mineral selenium (Se) is an essential element for human and animal nutrition. The addition of Se to the diet through dietary supplements or fortified food/feed is increasingly common owing to the often sub-optimal content of standard diets of many countries. Se supplements commercially available include the inorganic mineral salts such as sodium selenite or selenate, and organic forms such as Se-enriched yeast. Today, Se yeast is produced by several manufacturers and has become the most widely used source of Se for human supplementation and is also widely employed in animal nutrition where approval in all species has been granted by regulatory bodies such as the European Food Safety Authority (EFSA). Characterisation and comparison of Se-enriched yeast products has traditionally been made by quantifying total selenomethionine (SeMet) content. A disadvantage of this approach, however, is that it does not consider the effects of Se deposition on subsequent digestive availability. In this study, an assessment was made of the water-soluble extracts of commercially available Se-enriched yeast samples for free, peptide-bound and total water-soluble SeMet. Using LC-MS/MS, a total of 62 Se-containing proteins were identified across four Se yeast products, displaying quantitative/qualitative changes in abundance relative to the certified reference material, SELM-1 (P value <0.05; fold change ≥2). Overall, the study indicates that significant differences exist between Se yeast products in terms of SeMet content, Se-containing protein abundance and associated metabolic pathways.






Similar content being viewed by others
References
Zhang L, Hu B, Li W, Che R, Deng K, Li H, Yu F, Ling H, Li Y, Chu C (2013) OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol 201(4):1183–1191
Bierla K, Vacchina V, Szpunar J, Bertin G, Lobinski R (2008) Simultaneous derivatization of selenocysteine and selenomethionine in animal blood prior to their specific determination by 2D size-exclusion ion-pairing reversed-phase HPLC-ICP MS. J Anal At Spectrom 23(4):508–513
Wang W, Chen Z, Davey D, Naidu R (2009) Extraction of selenium species in pharmaceutical tablets using enzymatic and chemical methods. Microchim Acta 165(1):167–172
Barger J, Kayo T, Pugh T, Vann J, Power R, Dawson K, Weindruch R, Prolla T (2011) Gene expression profiling reveals differential effects of sodium selenite, selenomethionine, and yeast-derived selenium in the mouse. Genes Nutr 7(2):155–165
Zeng H, Jackson M, Cheng W-H, Combs G Jr (2011) Chemical form of selenium affects its uptake, transport, and glutathione peroxidase activity in the human intestinal Caco-2 cell model. Biol Trace Elem Res 143(2):1209–1218
Alzate A, Pérez-Conde MC, Gutiérrez AM, Cámara C (2010) Selenium-enriched fermented milk: a suitable dairy product to improve selenium intake in humans. Int Dairy J 20(11):761–769
Briens M, Mercier Y, Rouffineau F, Vacchina V, Geraert P-A (2013) Comparative study of a new organic selenium source v. seleno-yeast and mineral selenium sources on muscle selenium enrichment and selenium digestibility in broiler chickens. Br J Nutr 110(04):617–624
Xia Y, Hill KE, Byrne DW, Xu J, Burk RF (2005) Effectiveness of selenium supplements in a low-selenium area of China. Am J Clin Nutr 81(4):829–834
EFSA (2008) Selenium-enriched yeast as source for selenium added for nutritional purposes in foods for particular nutritional uses and foods (including food supplements) for the general population—scientific opinion of the panel on food additives, flavourings, processing aids and materials in contact with food. EFSA J 766:1–42
Murphy R (2013) Understanding different types of organic selenium. Feedstuffs 85(52):31–33
Hinojosa-Reyes L, Ruiz-Encinar J, Marchante-Gayun JM, Garcia-Alonso JI, Sanz-Medel A (2006) Selenium bioaccessibility assessment in selenized yeast after "in vitro" gastrointestinal digestion using two-dimensional chromatography and mass spectrometry. J Chromatogr A 1110(1–2):108–116
Encinar JR, Sliwka-Kaszynska M, Polatajko A, Vacchina V, Szpunar J (2003) Methodological advances for selenium speciation analysis in yeast. Anal Chim Acta 500(1–2):171–183
B’Hymer C, Caruso JA (2000) Evaluation of yeast-based selenium food supplements using high-performance liquid chromatography and inductively coupled plasma mass spectrometry. J Anal At Spectrom 15(12):1531–1539
Heras I, Palomo M, Madrid Y (2011) Selenoproteins: the key factor in selenium essentiality. State of the art analytical techniques for selenoprotein studies. Anal Bioanal Chem 400(6):1717–1727
Ganesh V, Hettiarachchy NS (2012) Nutriproteomics: a promising tool to link diet and diseases in nutritional research. Biochim Biophys Acta (BBA) - Proteins Proteomics 1824(10):1107–1117
El-Bayoumy K, Das A, Russell S, Wolfe S, Jordan R, Renganathan K, Loughran TP, Somiari R (2012) The effect of selenium enrichment on baker’s yeast proteome. J Proteome 75(3):1018–1030
Bierla K, Szpunar J, Yiannikouris A, Lobinski R (2012) Comprehensive speciation of selenium in selenium-rich yeast. TrAC Trends Anal Chem 41:122–132
Mester Z, Willie S, Yang L, Sturgeon R, Caruso J, Fernández M, Fodor P, Goldschmidt R, Goenaga-Infante H, Lobinski R, Maxwell P, McSheehy S, Polatajko A, Sadi B, Sanz-Medel A, Scriver C, Szpunar J, Wahlen R, Wolf W (2006) Certification of a new selenized yeast reference material (SELM-1) for methionine, selenomethinone and total selenium content and its use in an intercomparison exercise for quantifying these analytes. Anal Bioanal Chem 385(1):168–180
Ward P, Connolly C, Murphy R (2012) Accelerated determination of selenomethionine in selenized yeast: validation of analytical method. Biol Trace Elem Res 151(3):446–450
Dolan SK, Owens RA, O’Keeffe G, Hammel S, Fitzpatrick DA, Jones Gary W, Doyle S (2014) Regulation of nonribosomal peptide synthesis: bis-thiomethylation attenuates gliotoxin biosynthesis in Aspergillus fumigatus. Chem Biol 21(8):999–1012
Cox J, Mann M (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26(12):1367–1372
Perucchietti P, Litjens W (2012) Why check selenomethionine levels in selenium yeast? Allaboutfeednet 20(6):12–13
Polatajko A, Sliwka-Kaszynska M, Dernovics M, Ruzik R, Ruiz Encinar J, Szpunar J (2004) A systematic approach to selenium speciation in selenized yeast. J Anal At Spectrom 19(1):114–120
Goenaga Infante H, Hearn R, Catterick T (2005) Current mass spectrometry strategies for selenium speciation in dietary sources of high-selenium. Anal Bioanal Chem 382(4):957–967
McSheehy S, Kelly J, Tessier L, Mester Z (2005) Identification of selenomethionine in selenized yeast using two-dimensional liquid chromatography-mass spectrometry based proteomic analysis. Analyst 130(1):35–37
EFSA (2006) Opinion of the scientific panel on additives and products or substances used in animal feed on the safety and efficacy of the product Sel-Plex®2000 as a feed additive according to Regulation (EC) No 1831/2003. EFSA J 348:1–40
EFSA (2006) Opinion of the Panel on additives and products or substances used in animal feed (FEEDAP) on the safety and efficacy of the product Selenium enriched yeast (Saccharomyces cerevisiae NCYC R397) as a feed additive for all species in accordance with Regulation (EC) No 1831/2003. EFSA J :1-23
EFSA (2009) Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) on a request from the European Commission on the Safety and efficacy of SELSAF (Selenium enriched yeast from Saccharomyces cerevisiae CNCM I-3399) as feed additive for all species. EFSA J 992:1–24
Schrauzer GN (2003) The nutritional significance, metabolism and toxicology of selenomethionine. Adv Food Nutr Res 47:73–112
Rao Y, McCooeye M, Windust A, Bramanti E, D’Ulivo A, Mester Z (2010) Mapping of selenium metabolic pathway in yeast by liquid chromatography-orbitrap mass spectrometry. Anal Chem 82(19):8121–8130
Weekley CM, Harris HH (2013) Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem Soc Rev 42(23):8870–8894
Suhajda A, Hegoczki J, Janzso B, Pais I, Vereczkey G (2000) Preparation of selenium yeasts I. Preparation of selenium-enriched Saccharomyces cerevisiae. J Trace Elem Med Biol 14(1):43–47
Suzuki KT (2005) Metabolomics of selenium: Se metabolites based on speciation studies. J Health Sci 51(2):107–114
Hinojosa-Reyes L, Marchante-Gayon JM, Garcia Alonso JI, Sanz-Medel A (2006) Application of isotope dilution analysis for the evaluation of extraction conditions in the determination of total selenium and selenomethionine in yeast-based nutritional supplements. J Agric Food Chem 54(5):1557–1563
Moreda-Piñeiro J, Moreda-Piñeiro A, Romarís-Hortas V, Moscoso-Pérez C, López-Mahía P, Muniategui-Lorenzo S, Bermejo-Barrera P, Prada-Rodríguez D (2011) In-vivo and in-vitro testing to assess the bioaccessibility and the bioavailability of arsenic, selenium and mercury species in food samples. TrAC Trends Anal Chem 30(2):324–345
Rayman MP (2008) Food-chain selenium and human health: emphasis on intake. Br J Nutr 100(2):254–268
B’Hymer C, Caruso JA (2006) Selenium speciation analysis using inductively coupled plasma-mass spectrometry. J Chromatogr A 1114(1):1–20
Cabanero AI, Madrid Y, Camara C (2004) Selenium and mercury bioaccessibility in fish samples: an in vitro digestion method. Anal Chim Acta 526(1):51–61
Rayman MP (2004) The use of high-selenium yeast to raise selenium status: how does it measure up? Br J Nutr 92(04):557–573
Połatajko A, Banaś B, Encinar JR, Szpunar J (2005) Investigation of the recovery of selenomethionine from selenized yeast by two-dimensional LC–ICP MS. Anal Bioanal Chem 381(4):844–849
Gammelgaard B, Cornett C, Olsen Jr, Bendahl L, Hansen SH (2003) Combination of LC-ICP-MS, LC-MS and NMR for investigation of the oxidative degradation of selenomethionine. Talanta 59(6):1165–1171
Zembrzuska J, Matusiewicz H, Polkowska-Motrenko H, Chajduk E (2014) Simultaneous quantitation and identification of organic and inorganic selenium in diet supplements by liquid chromatography with tandem mass spectrometry. Food Chem 142:178–187
Tastet L, Schaumlöffel D, Bouyssiere B, Lobinski R (2006) Capillary HPLC–ICP MS mapping of selenocompounds in spots obtained from the 2-D gel electrophoresis of the water-soluble protein fraction of selenized yeast. Anal Bioanal Chem 385(5):948–953
Nesvizhskii AI, Vitek O, Aebersold R (2007) Analysis and validation of proteomic data generated by tandem mass spectrometry. Nat Methods 4(10):787–797
Baudouin-Cornu P, Lagniel G, Chédin S, Labarre J (2009) Development of a new method for absolute protein quantification on 2-D gels. Proteomics 9(20):4606–4615
McJury Richardson B, Soderblom EJ, Moseley MA (2013) Automated, reproducible, titania-based phosphopeptide enrichment strategy for label-free quantitative phosphoproteomics. J Biomol Tech 24(1):8–16
Asuero AG, Sayago A, Gonzalez AG (2006) The correlation coefficient: an overview. Crit Rev Anal Chem 36:41–59
Clough T, Key M, Ott I, Ragg S, Schadow G, Vitek O (2009) Protein quantification in label-free LC-MS experiments. J Proteome Res 8(11):5275–5284
EFSA (2013) Scientific opinion on the safety and efficacy of L-selenomethionine as feed additive for all animal species. EFSA J 11(5):3219
Bierla K, Bianga J, Ouerdane L, Szpunar J, Yiannikouris A, Lobinski R (2013) A comparative study of the Se/S substitution in methionine and cysteine in Se-enriched yeast using an inductively coupled plasma mass spectrometry (ICP MS)-assisted proteomics approach. J Proteome 87:26–39
Tastet L, Schaumloffel D, Lobinski R (2008) ICP-MS-assisted proteomics approach to the identification of selenium-containing proteins in selenium-rich yeast. J Anal At Spectrom 23(3):309–317
Goyco JA, Asenjo CF (1947) The net protein value of food yeast. J Nutr 33:593
Kieliszek M, Blazejak S (2013) Selenium: significance, and outlook for supplementation. Nutrition 29(5):713–718
Mapelli V, Hillestrom PR, Kapolna E, Larsen EH, Olsson L (2011) Metabolic and bioprocess engineering for production of selenized yeast with increased content of seleno-methylselenocysteine. Metab Eng 13(3):282–293
Rayman MP, Goenaga Infante H, Sargent M (2008) Food-chain selenium and human health: spotlight on speciation. Br J Nutr 100(2):238–253
Schrauzer GN (2006) Selenium yeast: composition, quality, analysis, and safety. Pure Appl Chem 78(1):105–109
Far J, Preud’homme H, Lobinski R (2010) Detection and identification of hydrophilic selenium compounds in selenium-rich yeast by size exclusion-microbore normal-phase HPLC with the on-line ICP-MS and electrospray Q-TOF-MS detection. Anal Chim Acta 657(2):175–190
Clark LC, Combs GF Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL Jr, Kim Park H, Sanders BB Jr, Smith CL, Taylor JR (1996) Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: a randomized controlled trial. JAMA 276(24):1957–1963
von der Haar T (2007) Optimized protein extraction for quantitative proteomics of yeasts. PLoS ONE 2(10):e1078
Mahn AV, Munoz MC, Zamorano MJ (2009) Discovery of biomarkers that reflect the intake of sodium selenate by nutritional proteomics. J Chromatogr Sci 47(9):840–843
Kanehisa M, Goto S (2000) KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 28(1):27–30
Priebe S, Linde J, Albrecht D, Guthke R, Brakhage AA (2011) FungiFun: a web-based application for functional categorization of fungal genes and proteins. Fungal Genet Biol 48(4):353–358
Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Frohlich K-U, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F (2009) Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 11(11):1305–1314
Dato L, Berterame N, Ricci M, Paganoni P, Palmieri L, Porro D, Branduardi P (2014) Changes in SAM2 expression affect lactic acid tolerance and lactic acid production in Saccharomyces cerevisiae. Microb Cell Factories 13:147
Malkowski MG, Quartley E, Friedman AE, Babulski J, Kon Y, Wolfley J, Said M, Luft JR, Phizicky EM, DeTitta GT, Grayhack EJ (2007) Blocking S-adenosylmethionine synthesis in yeast allows selenomethionine incorporation and multiwavelength anomalous dispersion phasing. Proc Natl Acad Sci 104(16):6678–6683
Luo J, Li Y-N, Wang F, Zhang W-M, Geng X (2010) S-Adenosylmethionine inhibits the growth of cancer cells by reversing the hypomethylation status of c-myc and H-ras in human gastric cancer and colon cancer. Int J Biol Sci 6(7):784–795
Chu J, Qian J, Zhuang Y, Zhang S, Li Y (2013) Progress in the research of S-adenosyl-l-methionine production. Appl Microbiol Biotechnol 97(1):41–49
Cherest H, Surdin-Kerjan Y, Exinger F, Lacroute F (1978) S-Adenosyl methionine requiring mutants in Saccharomyces cerevisiae: evidences for the existence of two methionine adenosyl transferases. Mol Gen Genet 163(2):153–167
Thomas D, Rothstein R, Rosenberg N, Surdin-Kerjan Y (1988) SAM2 encodes the second methionine S-adenosyl transferase in Saccharomyces cerevisiae: physiology and regulation of both enzymes. Mol Cell Biol 8(12):5132–5139
Thomas D, Surdin-Kerjan Y (1991) The synthesis of the two S-adenosyl-methionine synthetases is differently regulated in Saccharomyces cerevisiae. Mol Gen Genet 226(1–2):224–232
Ravanel S, Block MA, Rippert P, Jabrin S, Curien G, Rebeille F, Douce R (2004) Methionine metabolism in plants: chloroplasts are autonomous for de novo methionine synthesis and can import S-adenosylmethionine from the cytosol. J Biol Chem 279(21):22548–22557
Chiang PK (1998) Biological effects of inhibitors of S-adenosylhomocysteine hydrolase. Pharmacol Ther 77(2):115–134
Mapelli V, Hillestrøm PR, Patil K, Larsen EH, Olsson L (2012) The interplay between sulphur and selenium metabolism influences the intracellular redox balance in Saccharomyces cerevisiae. FEMS Yeast Res 12(1):20–32
Yadav AK, Desai PR, Rai MN, Kaur R, Ganesan K, Bachhawat AK (2011) Glutathione biosynthesis in the yeast pathogens Candida glabrata and Candida albicans: essential in C. glabrata, and essential for virulence in C. albicans. Microbiology 157(2):484–495
Collison EJ, Grant CM (2003) Role of yeast glutaredoxins as glutathione S-transferases. J Biol Chem 278(25):22492–22497
Izawa S, Maeda K, Miki T, Mano J, Inoue Y, Kimura A (1998) Importance of glucose-6-phosphate dehydrogenase in the adaptive response to hydrogen peroxide in Saccharomyces cerevisiae. Biochem J 330(Pt 2):811–817
Kumar A, Bachhawat AK (2010) OXP1/YKL215c encodes an ATP-dependent 5-oxoprolinase in Saccharomyces cerevisiae: functional characterization, domain structure and identification of actin-like ATP-binding motifs in eukaryotic 5-oxoprolinases. FEMS Yeast Res 10(4):394–401
Tomasi ML, Ramani K, Lopitz-Otsoa F, Rodríguez MS, Li TWH, Ko K, Yang H, Bardag-Gorce F, Iglesias-Ara A, Feo F, Pascale MR, Mato JM, Lu SC (2010) S-Adenosylmethionine regulates dual-specificity mitogen-activated protein kinase phosphatase expression in mouse and human hepatocytes. Hepatology 51(6):2152–2161
Acknowledgments
LC-MS facilities were funded by a competitive award from Science Foundation Ireland (12/RI/2346 (3)) to SD.
Conflict of Interest
SF, RM, CC and PW are employees of Alltech who retail selenium-enriched yeast as a commercial feed additive.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOCX 107 kb)
Rights and permissions
About this article
Cite this article
Fagan, S., Owens, R., Ward, P. et al. Biochemical Comparison of Commercial Selenium Yeast Preparations. Biol Trace Elem Res 166, 245–259 (2015). https://doi.org/10.1007/s12011-015-0242-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0242-6