Abstract
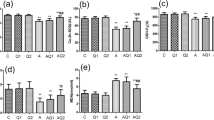
Acrylamide is an organic chemical which occurs in foods widespreadly consumed in diets worldwide. The purpose of this study was to evaluate the serum trace element levels (Fe, Cu, Zn, Mn, Cr, Se, Co, Ni, V, As, Mg, P, Li, K, Al) in Wistar rats exposed to acrylamide. Acrylamide was administered to the treatment groups at 2 and 5 mg/kg body weight (bw)/day via drinking water for 90 days. Inductively coupled plasma mass spectrometry was used for the determination of serum trace element concentrations. Serum Zn, Se, Co, V and Mg concentrations of 5 mg/kg bw/day acrylamide-treated male rats were lower, whereas serum As concentration was higher than the same parameters of the controls rats. Similarly, serum Zn, Se, Co, V and Mg concentrations were decreased in 5 mg/kg bw/day acrylamide-treated female rats compared with control rats. On the other hand, there were no significant differences between serum Fe, Cu, Mn, Cr, Ni, P, Li, K and Al concentrations of all groups. The results from this study provide evidence that dietary acrylamide intake adversely affects the serum trace elements status.




Similar content being viewed by others
References
Lineback DR, Coughlin JR, Stadler RH (2012) Acrylamide in fooods: a review of the science and future considerations. Annu Rev Food Sci Technol 3:15–35
Schettgen T, Rossbach B, Kütting B, Letzel L, Drexler H, Angerer J (2004) Determination of haemoglobinadducts of acrylamide and glycidamide in smoking and non-smoking persons of the general population. Int J Hyg Environ Health 207:531–539
Dearfield KL, Abernathy CO, Ottley MS, Brantner JH, Hayes PF (1988) Acrylamide: its metabolism, developmental and reproductive effects, genotoxicity, and carcinogenicity. Mutat Res 195:45–77
Friedman M (2003) Chemistry, biochemistry, and safety of acrylamide. A review. J Agr Food Chem 51:4504–4526
McCollister DD, Oyen F, Rowe VK (1964) Toxicology of acrylamide. Toxicol Appl Pharmacol 103:172–181
Yener Y, Dikmenli M (2009) Increased micronucleus frequency in rat bone marrow after acrylamide treatment. Food Chem Toxicol 47:2120–2123
Schwartz MK (1975) Role of trace elements in cancer. Cancer Res 35:3481–3487
Feng JF, Lu L, Zeng P, Yang YH, Luo J, Yang YW, Wang D (2012) Serum total oxidant/antioxidant status and trace element levels in breast cancer patients. Int J Clin Oncol 17(6):575–583
Özkaya MO, Nazıroğlu M, Barak C, Berkkanoglu M (2011) Effects of multivitamin/ mineral supplementation on trace element levels in serum and follicular fluid of women undergoing in vitro fertilization (IVF). Biol Trace Elem Res 139(1):1–9
Wasowicz W, Reszka E, Gromadzinska J, Rydzynski K (2003) The role of essential elements in oxidative stress. Comments on Toxicol 9:39–48
Nazıroğlu M (2009) Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem Res 34:2181–2191
Catalgol B, Ozhan G, Alpertunga B (2009) Acrylamide-induced oxidative stress in human erythrocytes. Hum Exp Toxicol 28(10):611–617
Yousef MI, El-Demerdash FM (2006) Acrylamide-induced oxidative stress and biochemical perturbations in rats. Toxicology 219:133–141
Smith MK, Zenick H, Preston RJ, George EL, Long RE (1986) Dominant lethal effects of subchronic acrylamide administration in the male Long-Evans rat. Mutat Res 173:273–277
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–31
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34(3):497–500
Gibson RS (1991) Trace element deficiencies in humans. Can Med Assoc J 145:231
Prasad AS (2009) Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care 12:646–652
Nazıroğlu M, Yıldız K, Tamtürk B, Erturan I, Flores-Arce M (2012) Selenium and psoriasis. Biol Trace Elem Res 150(1–3):3–9
Navarro-Alarcon M, Cabrera-Vique C (2008) Selenium in food and the human body: a review. Sci Total Environ 400:115–141
Oğuz AC, Naziroğlu M, Espino J, Bejarano I, González D, Rodríguez AB, Pariente JA (2009) Selenium modulates oxidative stress-induced cell apoptosis in human myeloid HL-60 cells through regulation of calcium release and caspase-3 and −9 activities. J Membr Biol 232(1–3):15–23
Dybing E, Sanner T (2003) Risk assessment of acrylamide in foods. Toxicol Sci 75:7–15
Allam A, El-Ghareeb AA, Abdul-Hamid M, Baikry A, Sabri MI (2011) Prenatal and perinatal acrylamide disrupts the development of cerebellum in rat: biochemical and morphological studies. Toxicol Ind Health 27:291–306
Vallee BL, Auld S (1990) Zinc coordination, function and structure of zinc enzymes and other proteins. Biochemistry 29:564–577
Levander OA (1977) Metabolic interrelationships between arsenic and selenium. Environ Health Perspect 19:159–164
Kajiyama H, Murase K, Miyazaki T, Isomoto H, Fukuda Y, Yamazawa N, Soda H, Takeshima F, Mizuta Y, Murata I, Kohno S (2001) Micronutrient status and glutathione peroxidase in bedridden patients on tube feeding. J Int Med Res 29:181–188
Scott N, Hatlelid KM, MacKenzie NE, Carter DE (1993) Reaction of arsenic (III) and arsenic (V) species with glutathione. Chem Res Toxicol 6:102–106
Mo J, Xia Y, Wade TJ, Schmitt M, Le XC, Dang R, Mumford JL (2006) Chronic arsenic exposure and oxidative stress: OGG1 expression and arsenic exposure, nail selenium, and skin hyperkeratosis in Inner Mongolia. Environ Health Persp 114:835–841
Flora SJ, Bhadauria S, Kannan GM, Singh N (2007) Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: a review. J Environ Biol 28:333–347
Yamasaki K, Sakuma Y, Sasaki J, Matsumoto K, Anzai K, Matsuoka K, Honda C, Tsukada M, Endo K, Enomoto S (2011) Biliary excretion of essential trace elements in rats under oxidative stress caused by selenium deficiency. Anal Bioanal Chem 401:2531–2538
Levander OA, Burk RF (1990) Selenium. In: Brown ML (ed) Present knowledge in nutrition, ed 6th edn. Nutrition Foundation, Washington, D.C, pp 268–273
Pilsner JR, Hall MN, Liu X, Ahsan H, Ilievski V, Slavkovich V, Levy D, Factor-Litvak P, Graziano JH, Gamble MV (2011) Associations of plasma selenium with arsenic and genomic methylation of leukocyte DNA in Bangladesh. Environ Health Persp 119:113–118
Zeng H (2001) Arsenic suppresses necrosis induced by selenite in human leukemia HL-60 cells. Biol Trace Elem Res 83:1–15
Meyerovitch J, Farfel Z, Sack J, Shechter Y (1987) Oral administration of vanadate normalizes blood glucose levels in streptozotocin-treated rats. Characterization and mode of action. J Biol Chem 262:6658–6662
Badmaev V, Prakash S, Majeed M (1999) Vanadium: a review of its potential role in the fight against diabetes. J Altern Complement Med 5:273–291
Guerrero-Romero F, Rodríguez-Morán M (2006) Hypomagnesemia, oxidative stress, inflammation, and metabolic syndrome. Diabetes Metab Res Rev 22:471–476
Weglicki WB, Mak IT, Kramer JH, Dickens BF, Cassidy MM, Stafford RE, Philips TM (1996) Role of free radicals and substance P in magnesium deficiency. Cardiovasc Res 31:677–682
Weglicki WB, Phillips TM, Freedman AM, Cassidy MM, Dickens BF (1992) Magnesium-deficiency elevates circulating levels of inflammatory cytokines and endothelin. Mol Cell Biochem 110:169–173
Naruszewicz M, Zapolska-Downar D, Kośmider A, Nowicka G, Kozłowska-Wojciechowska M, Vikström AS, Törnqvist M (2009) Chronic intake of potato chips in humans increases the production of reactive oxygen radicals by leukocytes and increases plasma C-reactive protein: a pilot study. Am J Clin Nutr 89:773–777
Prohaska JR (1991) Changes in Cu, Zn-superoxide dismutase, cytochrome c oxidase, glutathione peroxidase and glutathione transferase activities in copper-deficient mice and rats. J Nutr 121:355–363
Neve J (1991) The nutritional importance and pharmacologic effects of cobalt and vitamin B 12 in man. J Pharm Belg 46:271–280
Kawakami T, Hanao N, Nishiyama K, Kadota Y, Inoue M, Sato M, Suzuki S (2012) Differential effects of cobalt and mercury on lipid metabolism in the white adipose tissue of high-fat diet-induced obesity mice. Toxicol Appl Pharmacol 258:32–42
Abou-Zeina HAA, Zaghawa AA, Nasr SM, Keshta HGE (2008) Effects of dietary cobalt deficiency on performance, blood and rumen metabolites and liver pathology in sheep. Global Veterinaria 2:182–191
Kennedy DG, Kennedy S, Blanchflower WJ, Scott JM, Weir DG, Molloy AM, Young PB (1994) Cobalt-vitamin B12 deficiency causes accumulation of odd-numbered, branched-chain fatty acids in the tissues of sheep. Br J Nutr 86:67–76
Hokin B, Adams M, Ashton J, Louie H (2004) Comparison of the dietary cobalt intake in three different Australian diets. Asia Pac J Clin Nutr 13:289–91
Qureshi GA, Qureshi AA, Devrajani BR, Chippa MA, Syed SA (2008) Is the deficiency of vitamin B12 related to oxidative stress and neurotoxicity in Parkinson’s patients? CNS Neurol Disord Drug Targets 7:20–27
Birch CS, Brasch NE, McCaddon A, Williams JH (2009) A novel role for vitamin B(12): cobalamins are intracellular antioxidants in vitro. Free Radic Biol Med 47:184–188
Ling CT, Chow BF (1953) Effect of vitamin B12 on the levels of soluble sulfhydryl compounds in blood. J Biol Chem 202:445–456
Conflict of interest statement
The authors declare that there are no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yerlikaya, F.H., Yener, Y. The Dietary Acrylamide Intake Adversely Affects the Serum Trace Element Status. Biol Trace Elem Res 152, 75–81 (2013). https://doi.org/10.1007/s12011-013-9598-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-013-9598-7