Abstract
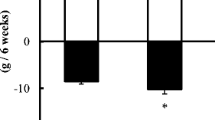
Cadmium is an environmental pollutant of increasing worldwide concern. It has been reported to be high in the soil where food crops are grown in some parishes of Jamaica. Surprisingly, no adverse effect of cadmium has been reported among the Jamaican population. However, phytic acid has also been shown to be high in some food crops grown in Jamaica. In this study, we evaluated the effects of phytic acid (1 %) and exercise on the metabolism of cadmium (5 mg cadmium/kg body weight) in rats. Five groups of rats were fed as follows: rats fed control diet, control diet supplemented with cadmium and subjected to exercise, control diet supplemented with phytic acid plus cadmium and subjected to exercise, control diet supplemented with cadmium plus phytic acid, and control diet supplemented with cadmium only. The animals were fed for 4 weeks and then sacrificed. Blood samples were collected for some biochemical assays. Percentage weight loss (28.42 %) was greatest in the group that had cadmium supplement only. The group fed control diet supplemented with cadmium only displayed increased liver enzymes and electrolytes except for the significant decrease in bicarbonate compared to other test groups. Similarly, blood urea nitrogen and uric acid were increased in the group fed cadmium supplement only compared to other test groups. Total cholesterol trended downwards in the test groups compared to control. These observations suggest that consumption of diet high in phytic acid with relatively high physical activity may be protective against the adverse effects of cadmium.







Similar content being viewed by others
References
Rimbach G, Pallauf J (1997) Cadmium accumulation, zinc status, and mineral bioavailability of growing rats fed diets high in zinc with increasing amounts of phytic acid. Biol Trace Elem Res 57(1):59–70
Agency for Toxic Substances and Disease Registry (1988) Toxicological profile for cadmium. US Public Health Service and US Environmental Protection Agency, Atlanta
National Sanitation Foundation (NSF) International (2003) Dietary Supplement-Standard 173 Metal Contamination Acceptance Levels. MI, USA
Omoruyi FO, Dilworth LL, Asemota HN, Jacobs H, Morrison EY (2004) An evaluation of cyanoglucoside, total phenol, protease inhibitors, phytic acid and zinc contents in some Caribbean food crops. Biosci Res Comm 15(3):225–229
Asagba SO (2009) Role of diet in absorption and toxicity of oral cadmium. Afr J Biotechnol 8(25):7428–7436
Pallauf J, Rimbach G (1997) Nutritional significance of phytic acid and phytase. Arch Tierernahr 50(4):301–319
Yoon JH, Thompson LU, Jenkins DJ (1983) The effect of phytic acid on in vitro rate of starch digestibility and blood glucose response. Am J Clin Nutr 38:835–842
Katayama T (1995) Effect of dietary sodium phytate on the hepatic and serum levels of lipids and on the hepatic activities of NADPH-generating enzymes in rats fed on sucrose. Biosci Biotechnol Biochem 59:1159–1160
Rundle A (2005) Molecular epidemiology of physical activity and cancer. Cancer Epidemiol Biomarkers Prev 14:227–236
Lalor GC, Raitray R, Simpson P, Vutchkov M (1998) Heavy metals in Jamaica, part 3: the distribution of cadmium in Jamaican soils. Rev Int Contam Ambien 14(1):7–12
Lalor GC (2008) Review of cadmium transfers from soil to humans and its health effect in the Jamaican environment. Sci Total Environ 400(1–3):162–172
Sokal RR, Rohlf FJ (1969) The principles and practice of statistics in biological research. Freeman and Company, San Francisco, pp 469–484
Elsenhans B, Strugala G, Schmann K (1999) Longitudinal pattern of enzymatic and absorptive functions in the small intestine of rats after short term exposure to dietary cadmium chloride. Arch Environ Contam Toxicol 36:341–346
Eriyamremu GE, Asagba SO, Onyeneke EC, Adaikpo MA (2005) Changes in carboxypeptidase A, dipeptidase and Na+/K+ATPase activities in the intestine of rats orally exposed to different doses of cadmium. BioMetals 18:1–6
Adachi K, Dote T, Dote E, Mitsui G, Kono K (2007) Strong acute toxicity, severe hepatic damage, renal injury and abnormal serum electrolytes after intravenous administration of cadmium fluoride in rats. J Occup Health 49(3):235–241
Gill TS, Epple A (1992) Impact of cadmium on the mummichog Fundulus heteroclitus and the role of calcium in suppressing heavy metal toxicity. Comp Biochem Physiol C 101(3):519–523
Verbost PM, Flik G, Lock RA, Wendelaar SE (1987) Cadmium inhibition of Ca2+ uptake in rainbow trout gills. Am J Physiol 253(2 Pt 2):R216–221
Johnson P (2002) Antioxidant enzyme expression in health and disease: effects of exercise and hypertension. Comp Biochem Physiol 133:493–505
Elsayed NM (2001) Antioxidant mobilization in response to oxidative stress: a dynamic environmental nutritional interaction. Nutrition 17:828–834
Turecki T, Ewan RC, Stahr HM (1994) Effect of phytic acid and calcium on the intestinal absorption of cadmium in vitro. Bull Environ Contam Toxicol 54:760–767
Turecki T, Ewan RC, Stahr HM (1995) Effect of dietary phytic acid and calcium on the availability of cadmium, zinc, copper, iron, and manganese to rats. Bull Environ Contam Toxicol 53:464–470
Markiewicz-Gorkai I, Antonowicz-Juchniewicz J, Pawlas K, Januszewska L (2007) Influence of physical training on level of oxidative damages in kidneys of rats intoxicated with cadmium as well as simultaneously cadmium and copper. Adv Clin Exp Med 16(4):479–491
Pincus MR, Sehaffner JR (1996) Assessment of liver function in clinical diagnosis and management by laboratory methods. Saunders, Philadelphia
Kowalczyk E, Kopff A, Fijalkowski P, Kopff M, Niewarok J, Blaszezyk J, Kedziora J, Tyslerowicz P (2003) Effect of anthocyanins on selected biochemical parameters in rats exposed to cadmium. Acta Biochim Pol 50(2):543–548
Guilhermino L, Soares AM, Carvalho AP, Lopes MC (1998) Effects of cadmium and parathion exposure on hematology and blood biochemistry of adult male rats. Bull Environ Contam Toxicol 60:52–59
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Daley, T., Omoregie, S.N., Wright, V. et al. Effects of Phytic Acid and Exercise on Some Serum Analytes in Rats Orally Exposed to Diets Supplemented with Cadmium. Biol Trace Elem Res 151, 400–405 (2013). https://doi.org/10.1007/s12011-012-9572-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9572-9