Abstract
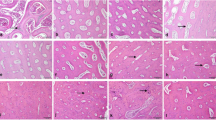
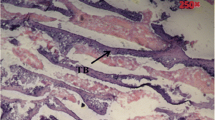
The aim of the present study was to find out the effects of boron on ostrich chicks fed with 0 mg/l, 100 mg/l, 200 mg/l, and 400 mg/l of additional boron in water. We measured bone mineral density (BMD), perimeter, length, weight, ash content of ostrich tibias, thickness of cortical bone, and diameter of the marrow cavity. We also analyzed the apoptosis status of paraffin sections using a TUNEL kit and examined serum levels of leptin and estradiol (E2). The results were dramatic. Compared with the control group, group C had a very high BMD. The serum levels of leptin in groups C and D were significantly higher than control values, and the levels of E2 fluctuated. The perimeter, length, weight, and ash content of ostrich tibias all increased significantly with increasing dosage of boron. The cross-section analysis revealed that the bone marrow cavity shifted closer to one side in group D, which was observed on a macro-scale. This shift may be related to the toxicity of excessive boron, as indicated by the apoptosis status. According to the present data, additional boron was helpful for ostrich chick bone development, and 200 mg/l supplement boron in the drinking water appeared to be the most beneficial.





Similar content being viewed by others
References
Linnaeus C (1758) Systema naturae (Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. TomusI. Editio decima, reformata) Vol “S. pedibus didactylis”. Holmiae, Stockholm, p 155
Aire TA, Soley JT, Groenewald HB (2003) A morphological study of simple testicular cysts in the ostrich (Struthio camelus). Res Vet Sci 74:153–162
Al-Nasser A, Al-Khalaifa H, Holleman K et al (2003) Ostrich production in the arid environment of Kuwait. J Arid Environ 54:219–224
Shi FP (2006) Young ostrich toe leg disease comprehensive prevention and control. J Anim Sci Vet Med 25:60
Wilson J, Ruszler P (1998) Long term effects of boron on layer bone strength and product ion parameters. Br Poult Sci 3:11–15
Devirian T, Volpe S (2003) The physiological effects of dietary boron. Crit Rev Food Sci Nutr 43:219–231
Volpe S, Taper L (1993) The relationship between boron and magnesium status and bone mineral density in the human: a review. Meacham SM Agnes Res 2:291–296
Rainey C, Nyquist L (1998) Relationships between boron concentrations and trout in the Firehole River, Wyoming. Biol Trace Elem Res 66:167–184
Fort DJ, Propst TL, Stover EL et al (1998) Adverse reproductive and developmental effects in Xenopus from insufficient boron. Biol Trace Elem Res 66:237–259
Sheng MH-C, Taper LJ, Veit H et al (2001) Dietary boron supplementation enhances the effects of estrogen on bone mineral balance in ovariectomized rats. Biol Trace Elem Res 81:29–45
Wester RC, Hui X, Maibach HI et al (1998) In vivo percutaneous absorption of boron as boric acid, borax, and disodium octaborate tetrahydrate in humans. Biol Trace Elem Res 66:101–109
Bajoria R, Sooranna SR, Chatterjee R (2007) Leptin and bone turnover in monochorionic twins complicated by twin–twin transfusion syndrome. Osteoporos Int 18:193–200
Wang Y (2008) The mechanical research about the influence of boron to the hypothalamic–pituitary–ovary axis of ostrich chicks, in College of Animal Science and Veterinary Medicine. Huazhong Agricultural University, Wuhan, pp 26–27
Cilliers SC, Hayes JP, Chwalibog A et al (1998) Determination of energy, protein and amino acid requirements for maintenance and growth in ostriches. Anim Feed Sci Technol 72:283–293
Lanza M, Fasone V, Galofaro V et al (2004) Citrus pulp as an ingredient in ostrich diet: effects on meat quality. Meat Sci 68:269–275
Liang CZ, Yang YJ (1996) Ostrich-raising management and disease control. Beijing Agricultural University Press, Beijing, pp 42–56
Cao JX (2002) Rats femoral ash and bone calcium content is determined. Chin J Environ Occup Med 19:188–189
Yeh F, Am G, Sm W (2006) Children who experience their first fracture at a young age have high rates of fracture. Osteoporos Int 17:267
Toshitaka N (2000) Concept of osteoporosis and its change (Japan). Curr Ther 18:180–184
Dan P (2008) Concept osteoporosis. Available at http://www.wikinvest.com/concept/Osteoporosis
Li JS (2003) Leg disease of ostrich chicks. Agric Sci Technol Inform 11:31
Liu ZX, Tang XL (2001) Ostrich chick leg disease diagnosis. Shandong Poult 1:24–25
Qiang AZ, Ding YB (2001) Prevention brood period ostrich leg bending deformation disease. Ningxia Forest Technol 4:35
Wang WJ (2002) Ostrich nonnutritive embryonic disease. Henan J Anim Husbandry Vet Med 23:42
Sun E, Zheng C (2005) Influence factors of young ostrich survival rate. Jilin Anim Husbandry Vet 3:58
Bronner F, John PB, Lawrence GR et al (2002) Metals in bone: aluminum, boron, cadmium, chromium, lead, silicon, and strontium, in principles of bone biology, 2nd edn. Academic, San Diego, pp 359–369
Nielsen FH (2009) Micronutrients in parenteral nutrition: boron, silicon, and fluoride. Gastroenterology 137:S55–S60
Cooper C, Fall C, Egger P et al (1997) Growth in infancy and bone mass in later life. Ann Rheum Dis 56:17
Cooper C, Cawley M, Bhalla A et al (1995) Childhood growth, physical activity, and peak bone mass in women. J Bone Miner Res 10:940
Duppe H, Cooper C, Gardsell P et al (1997) The relationship between childhood growth, bone mass, and muscle strength in male and female adolescents. Calcif Tissue Int 60:405
Uysal T, Ustdal A, Sonmez MF et al (2009) Stimulation of bone formation by dietary boron in an orthopedically expanded suture in rabbits. Angle Orthod 79:984–990
Hakki SS, Bozkurt BS, Hakki EE (2010) Boron regulates mineralized tissue-associated proteins in osteoblasts (MC3T3-E1). J Trace Elem Med Biol 24:243–250
Nielsen FH, Stoecker BJ (2009) Boron and fish oil have different beneficial effects on strength and trabecular microarchitecture of bone. J Trace Elem Med Biol 23:195–203
McCoy H, Kenney M, Montgomery C et al (1994) Relation of boron to the composition and mechanical properties of bone. Environ Health Perspect 1:49–53
Fail P, Chapin R, Price C et al (1998) General, reproductive, developmental, and endocrine toxicity of boronated compounds. Reprod Toxicol 12:1–18
Sabuncuoglu BT, Kocaturk PA, Yaman O et al (2006) Effects of subacute boric acid administration on rat kidney tissue. Clin Toxicol (Phila) 44:249–253
Ku WW, Chapin RE, Wine RN et al (1993) Testicular toxicity of boric acid (BA): relationship of dose to lesion development and recovery in the F344 rat. Reprod Toxicol 7:305–319
Akcakus M, Kurtoglu S, Koklu E et al (2007) The relationship between birth weight leptin and bone mineral status in newborn infants. Neonatology 91:101–106
Steppan C, Dtckl CF (2000) Leptin is a potent stimulator of bone growth in ob mice. Regul Pept 92:73278
Hamrick M, Pennington C, Newton D et al (2004) Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 34:376–383
Iwamoto I, Fujino T, Douchi T (2004) The leptin receptor in human osteoblasts and the direct effect of leptin on bone metabolism. Gynecol Endocrinol 19:23–25
Milton L (2002) Awakening the silent osteoporosis market. Medical marketing & media. Available at http://findarticles.com/p/articles/mi_hb3272/is_200204/ai_n7972338
Yamaguchi M, Kitajima T (1991) Effect of estrogen on bone metabolism in tissue culture: enhancement of the steroid effect by zinc. Res Exp Med 191:145–154
Cooper C, Stakkestad JA, Radowicki S et al (1999) Matrix delivery transdermal 17β-estradiol for the prevention of bone loss in postmenopausal women. Osteoporos Int 9:358–366
Sowers MR, Finkelstein JS, Ettinger B et al (2003) The association of endogenous hormone concentrations and bone mineral density measures in pre- and perimenopausal women of four ethnic groups: SWAN. Osteoporos Int 14:44–52
Poet JL, Galinier PA, Tonolli SI et al (1993) Lumbar bone mineral density in anorexia nervosa. Clin Rheumatol 12:236–239
Gallardo-Williams MT, Maronpot RR, Turner CH et al (2003) Effects of boric acid supplementation on bone histomorphometry, metabolism, and biomechanical properties in aged female F-344 rats. Biol Trace Elem Res 93:155–169
Disilvio L, Jameson J, Gamie Z et al (2006) In vitro evaluation of the direct effect of estradiol on human osteoblasts (HOB) and human mesenchymal stem cells (h-MSCs). Injury 37:S33–S42
Acknowledgments
This work was supported by the National Natural Science Foundation of China No. 30972152 and No. 39970547, and Specialized Research Fund for the Doctoral Program of China Higher Education No. 200805040023.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, J., Peng, K., Jin, E. et al. Effect of Additional Boron on Tibias of African Ostrich Chicks. Biol Trace Elem Res 144, 538–549 (2011). https://doi.org/10.1007/s12011-011-9024-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-9024-y