Abstract
Fructooligosaccharides (FOS) and levan attract much attention due to a wide range of applications in food technology and pharmaceutical and cosmetic industry. Bacillus licheniformis ANT 179, isolated from Antarctica soil, produced levansucrase and levan in a medium containing sucrose as carbon substrate. In this study, characterization of levansucrase and production of short-chain FOS and levan were investigated. Temperature and pH optimum of the enzyme were found to be 60 °C and pH 6.0, respectively. The optimization of fermentation conditions for levan production using sugarcane juice by response surface methodology (RSM) was carried out. Central composite rotatable design was used to study the main and the interactive effects of medium components: sugarcane juice and casein peptone concentration on levan production by the bacterium. The optimized medium with sugarcane juice at 20 % (v/v) and casein peptone at 2 % (w/v) was found to be optimal at an initial pH of 7.0 and incubation temperature of 35 °C for 48 h. Under these conditions, the maximum levan concentration was 50.25 g/L on wet weight basis and 16.35 g/L on dry weight basis. The produced inulin type FOS (kestose and neokestose) and levan were characterized by Fourier transform infrared spectroscopy (FT-IR) and nuclear magnetic resonance (NMR) analysis. The study revealed that the levansucrase could form FOS from sucrose. The locally available low-cost substrate such as sugarcane juice in the form of a renewable substrate is proposed to be suitable even for scale-up production of enzyme and FOS for industrial applications. The levan and FOS synthesized by the bacterium are suitable for food applications and biomedical uses as the bacterium has GRAS status and devoid of endotoxin as compared to other Gram-negative bacteria.
Similar content being viewed by others
Introduction
Levansucrase (EC 2.4.1.10) is a multifunctional enzyme that hydrolyzes sucrose into glucose and fructose apart from synthesis of fructans by direct transfer of fructosyl moiety to sucrose, thereby forming fructooligosaccharides (FOS) up to a chain length of 10 fructose moieties. The production of levan of β(2→6)-linked fructose type with β(2→1)-linked branching points is also catalyzed by these enzymes [1]. Levansucrase is an extracellular bacterial enzyme which belongs to glycoside hydrolase family 68 (GH68).
FOS are fructans with a chain extending up to ten fructose units, with one to four fructose molecules linked to sucrose by β(2→1) linkages. The FOS are non-digestible oligosaccharides widely used as low calorific artificial sweeteners and proven prebiotics [2]. The FOS obtained from sucrose using levansucrase is predictable, whereas in case of inulinase, the oligosaccharide production might vary due to the endoinulinase or exoinulinase nature of the inulinase. Hence, levansucrase for production of FOS attracts certain advantage [3]. Transfructosylating enzymes capable of producing FOS from sucrose are rare when compared to inulinase-producing bacteria. Park et al. [4] reported production of FOS of GF5 and GF6 using levansucrase from Bacillus macerans EG-6 on 50 % (w/v) sucrose as substrate.
Microbial levan is a fructan type of polymer comprising of β-(2,6)-fructofuranosyl units linked with multiple side chains [5]. Production of microbial levan is achieved using sucrose-based substrates with high C/N ratio. The levan finds a variety of applications in food industry and biomedical technologies [6]. Srikanth et al. [5] reported levan production (13.25 g/L) by Acetobacter xylinum NCIM 2526 with antioxidant and anti-inflammatory properties using a medium containing a high level of sucrose in a batch fermentation. Microbial production of levan is dependent on various factors such as temperature, pH, incubation period, carbon, and nitrogen source. Response surface methodology (RSM) has emerged as a useful tool for optimization of factors responsible for the desirable effect on production of levan from Zymomonas mobilis, Pseudomonas fluorescence, and B. licheniformis [7].
Levan production from sucrose by Antarctica bacteria is usually reported as a biochemical property. In recent years, Antarctica and other marine bacteria belonging to genera such as Bacillus, Plomococcus, and Pseudoalteromonas have been shown to produce exopolysaccharides. The significance of exopolysaccharide production in marine bacteria is attributed to nutrient entrapment, adhesion to solid surfaces, the ability to withstand extremes of temperatures and desiccation, and function as a carbon source [8]. However, the Antarctic bacterial communities are known to be unique and diverse in terms of their physiological properties. Bacillus sp. is one of the predominant genera that produce a variety of macromolecules and biopolymers as an adaptation strategy to withstand the extremes of temperatures [9]. In particular, the bacterium B. licheniformis ANT 179 was found to produce levan; further studies revealed that the bacterium produces levansucrase as an extracellular enzyme, and further, it was found to synthesize FOS under in vitro conditions. The levansucrase from culture broth was purified, characterized, and used for the synthesis of FOS. The effect of nutritional and other physicochemical parameters on the FOS and levan production was investigated. Levan production using low-cost carbohydrate such as sugarcane juice by one-factor-at-a-time methodology (including carbon source) was attempted followed by statistical optimization along with FOS and levan product confirmation by thin-layer chromatography (TLC), Fourier transform infrared (FT-IR), and nuclear magnetic resonance (NMR) analysis.
Materials and Methods
Sample Collection
The soil samples were collected from Antarctica between January 20 and February 10, 1994 during the Fourteenth Indian Summer Scientific Expedition. The geographic coordinates of the sampling area are 70° 45′ S and 11° 46′ E. The samples were transported to the laboratory at 4 °C and stored in −20 °C.
Isolation and Characterization of B. licheniformis ANT 179 Isolate
The bacterium was isolated from a soil sample collected around Maitri station in Antarctica. Tenfold serial dilutions of soil samples in sterilized normal saline were spread on nutrient agar plates containing peptone 5.0 g, yeast extract 2.0 g, beef extract 1.0 g and distilled water 1000 ml. The bacterial colonies appeared on nutrient agar plates, after incubation at 10–20 °C for 48 h. Discrete colonies were picked and inoculated in nutrient broth and grown at 20 °C. The isolate formed smooth, round mucoid colonies and appeared cream in color, while cells were Gram-positive and small rods. Our earlier investigations on Antarctic isolates indicated the occurrence of Pseudomonas and Bacillus as predominant species which hydrolyze denatured protein and water-soluble lipase substrates and tributyric acid [10]. The strain was identified by sequencing the 1.4 kb of 16S rRNA gene amplified using universal forward primer 5′ AGAGTTTGATCCTGGCTAG 3′ and reverse primer 5′ AAGGAGGTGATCCAGCC 3′. The 16S rRNA gene sequence analysis was carried out using NCBI-BLAST homology search (National Centre for Biotechnology Information, http://www.ncbi.nml.nih.gov) program and identified the bacterium as Bacillus licheniformis. The nucleotide sequence has been deposited in the NCBI database under the Genbank accession number JQ000031.1. The glycerol stock culture maintained at −20 °C was used for inoculum preparation.
Media Components
Carbon sources such as fructose, galactose, lactose, maltose, xylose, and nitrogen sources such as casein peptone and protease peptone were procured from HiMedia (Mumbai, India). Sucrose, sodium chloride, yeast extract, phenol, HCl, H2SO4 and ethanol were procured from SD Fine Chemicals (Mumbai, India). The standard Z. mobilis derived levan used in this study was procured from Sigma-Aldrich (Mumbai, India).
Levansucrase Production, Purification, and Characterization
All the procedures described below were performed at 4 °C. The B. licheniformis ANT 179 was grown in NB medium supplemented with 5 % sucrose (w/v) at 35 °C for 48 h. The cell-free culture supernatant was obtained by centrifugation at 6800×g for 20 min and concentrated at −20 °C in a lyophilizer (Labconco, Freeze Zone). Enzyme from 50 ml concentrated cell-free supernatant was precipitated by addition of an equal volume of 80 % cold ethanol and kept at 4 °C for overnight. The supernatant was also saturated with approximately 80 % ammonium sulfate under stirring at 4 °C. The protein fraction was collected by centrifugation at 6800×g for 15 min at 4 °C. The pellet obtained by ethanol and ammonium sulfate fractionations was dissolved in 15 ml of 50 mM citrate buffer pH 6.0. The enzyme solution was applied to a Sephadex G-100 column (2.0 × 85 cm) previously equilibrated with 50 mM phosphate buffer pH 6 for further purification. Bound proteins were eluted from the column at a flow rate of 30 ml/h in the same buffer, and fractions (3 ml each) were collected.
The gel-eluted fractions were assayed for protein and levansucrase activity. Enzyme activity was performed by adding 0.5 ml of crude enzyme to 0.5 ml of 30 % (w/v) sucrose dissolved in phosphate buffer (50 mM, pH 6.0) followed by incubation at 30 °C for 30 min. The activity was measured as the amount of glucose released using a glucose oxidase kit (Merck, India). The absorbance was measured at 540 nm, and the amount of glucose released from sucrose was calculated using d-glucose as standard. One unit of levansucrase was defined as the amount of enzyme that produced 1.0 μmol of glucose per minute under standard conditions. The molecular mass of levansucrase was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by comparing the size of the major band with standard protein markers (Puregene) according to the method of Laemmli [11]. Proteins in the polyacrylamide gel were visualized by Coomassie brilliant blue R-250 staining.
Effect of Temperature and pH on Enzyme Activity
Optimum temperature for the activity of levansucrase was determined by incubating the reaction mixture at 30.0–100.0 °C in 50 mM phosphate buffer, pH 6.0, using sucrose as the substrate (30 % w/v). Temperature stability of the enzyme was tested by pre-incubation for 30 min at different temperatures in the range 30.0–100.0 °C at pH 6.0, and the relative activity of levansucrase was measured. The pH stability of levansucrase was assessed by pre-incubating the enzyme for 12 h with buffered sucrose ranging from pH 4.0 to 12.0, pH 4.0 and 5.0 (sodium acetate buffer), pH 6.0, pH 7.0, pH 8.0 (phosphate buffer), and pH 9.0, 10.0, 11.0, and 12.0 (glycine–NaOH buffer) at 4 °C. The residual enzyme activity was determined under standard assay conditions. The levansucrase activity of the pre-incubated sample at 4 °C was taken as 100 %.
Effect of Metal Ions and Other Chemicals on Levansucrase Activity
The effect of metal ions and other compounds on enzyme activity was tested by pre-incubating the enzyme in the presence of 50 mM phosphate buffer (pH 6.0) containing 1 mM and 10 mM of Ba2+, Ca2+, Cu2+, K+, Li+, Mn2+, Mg2+, Na+, Ni2+, Zn2+, NH4Cl, EDTA, and sodium acetate at room temperature for 1 h. The reaction was started by addition of 30 % (w/v) buffered sucrose to determine the residual activity. All the experiments were performed in triplicate, and the error was expressed as the standard deviation of the three measurements.
Production of Fructooligosaccharides by Levansucrase
To a solution of 30 % (w/v) sucrose in 50 mM phosphate buffer (pH 6.0), purified levansucrase was added and the mixture incubated at 37 °C with gentle stirring for 12 h. The products of enzyme hydrolysis were purified using an equal volume of 100 % (v/v) ethanol and centrifuged at 10,000 rpm for 10 min to precipitate the high molecular weight compounds. The supernatant was filtered with 0.45-μm nylon syringe filters and conveniently diluted for analysis.
Production of Levan by Levansucrase
To a solution of 30 % (w/v) sucrose in 50 mM phosphate buffer (pH 6.0), ethanol-fractionated levansucrase 30 % (v/v) was added and the mixture was incubated at 35 °C for 12 h. Levansucrase was obtained from fermentation of B. licheniformis ANT 179 in different media, viz. nutrient broth, nutrient broth (2×), and nutrient broth (2×) containing 5 % (w/v) sucrose incubated at three different temperatures, i.e., 30, 35, and 40 °C. Levan synthesis was observed as turbidity and was measured at 540 nm using UV–vis spectrophotometer (Shimadzu). The amount of levan formed was calculated from a standard graph using the purified levan as the internal standard [12]. One unit of levan forming activity was expressed as the amount of enzyme required to form 1 μg of levan per minute under experimental conditions.
Inoculum Preparation and Production of Levan
The inoculum of B. licheniformis ANT 179 was prepared by transferring a loopful of the inoculum into 30 ml of sterile production medium in a 100-ml Erlenmeyer flask. The inoculated medium was kept for 24 h at 28 ± 2 °C under static conditions. Production of the polymer was carried out in 250-ml capacity Erlenmeyer flasks containing 100-ml medium. The production medium was inoculated with 3 % (v/v) of 24-h-old B. licheniformis ANT 179 seed culture. The inoculated flasks were kept under static condition at 35 ± 2 °C. To study the effect of aeration on the production of levan, the media containing sucrose (50 g/L), protease peptone (20 g/L), and yeast extract (2 g/L) pH 7.0 were inoculated with 3 % (v/v) of overnight grown culture and agitated at 200 rpm. The effect of incubation time on levan production was studied by incubation of culture for 24, 48, 72, and 96 h.
Effect of Temperature and pH on Growth and Levan Production
To study the optimum incubation temperature for growth of levan-producing bacteria and levan production, B. licheniformis ANT 179 was incubated at different temperatures such as 4, 15, 28, 35, and 45 °C at pH 6.0, 6.5, 7.0, 7.5, and 8.0. Cell density was measured by reading the OD at 600 nm in a UV–vis spectrophotometer (Shimadzu, Japan).
Selection of Carbon and Nitrogen Source for Levan Production
The influence of different carbon sources such as glucose, maltose, lactose, galactose, xylose, and starch was studied at 2 % (w/v) instead of 2 % (w/v) sucrose in the media for production of levan. Cost-effective regionally available carbon sources such as sugarcane juice and molasses were also studied at 20 % (v/v) by replacing sucrose 50 % (w/v) in the basal medium. The effect of nitrogen sources on levan production was also studied using protease peptone and casein peptone at 2 %. The pH of the media was adjusted to 7.0 by the addition of 1 N NaOH prior to autoclaving. Overnight cultures were inoculated at 3 % (v/v), and fermentation was carried out at 35 °C for 72 h under agitation at 200 rpm in 250-ml Erlenmeyer flasks containing 100-ml media.
Central Composite Rotatable Design (CCRD)
To maximize the levan production from B. licheniformis ANT 179, a combination of suitable physical parameters was identified based on “one-at-a-time” approach followed by CCRD using RSM (Minitab software, Version, 14). CCRD is an experimental design which enables to fit a first- or second-order polynomial by a least significance technique. This design facilitates in locating the optimum point within the unknown region of interest and also ensures the uniformity of the magnitude of prediction error for all points at the same radial distance from the center point [13]. The CCRD for three independent variables such as sugarcane juice (X 1, %), casein peptone (X 2, %), and pH (X 3) each at three levels leading to a total number of 20 experiments was employed for optimization. Each variable was studied at two different levels (−1, +1) and center point (0) which is the midpoint of each factor range. In developing the regression equation, the test factors were coded according to the following equation:
where x is the dimensionless coded value, X i is the actual value of variables, X 0 is the actual value of variables at the center point, and ∆X is the step change value. The experimental results were fitted with a second-order polynomial function:
where Y is the predicted response, b 0 the model constant, b 1, b 2, and b 3 the linear coefficient, b 11, b 22, and b 33 the squared coefficient, and b 12, b 13, and b 23 the interaction coefficient.
Data Analysis
Minitab software, version 14 [14], was used for the data analysis. The response surface model graphs were used to identify the effects of linear, quadratic, and interactive terms of the independent variables on the chosen dependent variables. To validate the model, the average of each response (Levan yield) was determined from the completely optimized medium composition in duplicates. The statistical significance of the model was checked by Fischer’s F test, and the level of significance was given as p value.
Purification of Levan
After incubation for 72 h, the cell biomass was separated by centrifugation at 12,000 rpm for 15 min and the levan from supernatant was precipitated using absolute ethanol at 4 °C, thrice the volume of supernatant. The precipitated levan pellet was resuspended in distilled water containing 3 % (w/v) pepsin and incubated at 37 °C for 24 h. The solution was heated at 100 °C for 10 min and centrifuged at 12,000 rpm for 15 min to remove the denatured protein. The supernatant obtained was dialyzed against distilled water for five times and lyophilized.
TLC and HPLC Analysis of FOS and Levan
TLC of FOS and levan was performed with Silica gel 60 plates (Merck, Germany). The solvent system for separation of oligosaccharides was a mixture of n-butanol/ethanol/water (5:3:2, v/v/v). Sugars were detected by spraying the TLC sheets with a solution of 5 % (v/v) of sulfuric acid in methanol and subsequent heating at 120 °C for 3–5 min. The solvent system for levan analysis was a mixture of butanol/propanol/water (1:3:1, v/v/v). Sugars were detected by spraying the TLC sheets with a solution of 5 % (v/v) of sulfuric acid in methanol and subsequent heating at 100 °C until spots appeared.
FT-IR and NMR Analysis
The FT-IR spectra of the oligomers were obtained using a Shimadzu FT-IR, Model IR-Affinity-1. The spectra were recorded over a wavelength of 400 to 4000 cm−1. The levan produced was also subjected to FT-IR analysis and compared with standard levan. The spectra were recorded using the potassium bromide pellet method. Twenty-five scans were averaged to get the spectra. The IR spectra were recorded with 16 cm−1 resolution, and analysis of the spectra was carried out using software. 13C NMR spectra of the polysaccharide levan and fructooligosaccharides synthesized by levansucrase through the use of sucrose substrate were recorded with Bruker DSX 300 spectrometer at 100.64 MHz employing standard Bruker NMR software. The solvent used for levan was deuterated chloroform. Deuterated water was used for FOS.
Results and Discussions
Characterization of Levansucrase
The purified levansucrase used in the present study showed a single major band of approximately 30 kDa on 10 % SDS-PAGE gels (Fig. 1); previous reports on levansucrase indicated similar molecular weight [15]. The optimal conditions for enzyme activity in terms of temperature and pH for the enzyme were investigated based on the glucose released by the enzyme from sucrose. The influence of temperature on levansucrase activity was examined at pH 6.0, and the optimum temperature was found to be 60 °C. The enzyme activity at 50 °C was 50–70 % and less than 40 % above 60 °C. The temperature stability drastically reduced on incubation above 50 °C for 20 min (Fig. 2a, b). Velaquez-Hernandez et al. [16] reported that majority of levansucrases produced by Bacillus sp. are active at 30 °C, while Ammar et al. [15] reported a levansucrase active at 60 °C. The levansucrase from B. licheniformis ANT 179 exhibited 50 % of its activity in a pH range of 4.0–12.0 with the maximum activity at pH 6.0 (Fig. 3). The relative activity of enzyme increased above pH 4.0 and retained 50 % of its activity over the pH range 4.0–12.0 even after 18-h incubation. Earlier reports indicated optimum activity of levansucrase at pH 6.0 from Bacillus subtilis BB04 while at pH 5.6 and more than 7.6 in an enzyme from Bacillus megaterium [17]. The effect of metal ions and other chemicals at 1 and 10 mM concentrations on the activity of levansucrase was investigated, and Ba2+, Ca2+, Cu2+, K+, Li+, Mn2+, Mg2+, Na+, Ni2+, Zn2+, NH4Cl, EDTA, and sodium acetate, in 50 mM phosphate buffer (pH 6.0), were studied at room temperature. At 1 mM concentration, the enzyme activity was enhanced in the presence of all the metal ions and chemicals tested when at 10 mM concentrations only Ba2+, Cu2+, Ni2+, K+, Li+, Mg2+, and sodium acetate enhanced the enzyme activity. On the other hand, Ca2+, K+, Mn2+, Na+, Zn2+, NH4Cl, and EDTA were found to inhibit the activity (Table 1).
FOS and Levan Synthesis by Levansucrase
The levansucrase is known to display three types of activity based on the prevailing condition for the enzyme in a reaction titer milieu: transfer of fructosyl moiety of sucrose to the growing polymer chain resulting in formation of levan while glucose is released as by-product, synthesis of short-chain FOS by transfer of the fructosyl group to acceptors such as glucose and fructose, and the hydrolysis of sucrose resulting in release of fructose [16]. FOS synthesized by the enzyme was detected by TLC. Lu et al. [18] reported the production of recombinant levansucrase from B. licheniformis 8-37-0-1 which could catalyze versatile transfructosylation reactions and also produce a novel levan (41.7 g/L).
The media were prepared containing 30 % (w/v) sucrose, and the flasks were incubated for 24 and 48 h for levansucrase production. Levansucrase production was found to be higher from culture supernatant obtained after 48 h fermentation when compared to the culture supernatant obtained after 24 h fermentation. The initial concentration of sucrose was 30 %, and levan production was highest (32.0 g/L) on incubation with levansucrase precipitated from nutrient broth (2×) medium incubated at 30 and 35 °C for 48 h (Table 2). The difference in levan production may be due to the effect of media components on levansucrase production. Abdel-Fattah and Esawy [19] tested the effect of different nitrogen sources and reported that baker’s yeast at 2 % (w/v) and addition of 0.15 g/L MgSO4 gave the highest levansucrase activity. Even though there was levan production up to 32.0 g/L from sucrose using fractionated levansucrase, media optimization for production of levan from low-cost regional substrate by fermentation of B. licheniformis ANT 179 was carried out.
Levan Production by Fermentation
The effect of incubation time on the levan production was studied at 24, 48, 72, and 96 h. It was found that the maximum amount of levan was produced when incubated for 48 h, while production was lower after 24, 72, and 96 h incubation periods. The decrease in levan production after 48 h incubation is attributed to product inhibition. As hydrolase activity is well documented for levansucrases at maximum levan concentrations, if the fermentation is allowed to continue, there will be a decrease in high molecular weight levan concentration and an increase in the amount of low molecular weight levan, oligos, and even monomers. Under some conditions, the increase in oxygen availability would allow production of a greater biomass but may have an adverse effect on the amount of levan produced. In our study, due to continuous agitation, oxygen supply was provided to bacterial cells, which facilitated their growth and enhanced the productivity. The effect of different temperatures at 4.0, 15.0, 28.0, 30.0, 35.0, and 45.0 °C on levan production was studied. The growth of bacteria was observed at 620 nm OD at temperatures 4.0, 15.0, 28.0, and 30.0 °C at 18, 24, and 48 h (supplementary information). The production of levan at 4.0 and 15.0 °C was negligible. Synthesis reached a maximum at 35 °C, while an increase in 35 °C decreased levan production (supplementary information). Hence, further experiments were carried out at temperature 35 °C and maximum productivity of levan was obtained after 72 h incubation.
Effect of Carbon and Nitrogen Sources on Levan Production
Sugars such as galactose, lactose, maltose, sucrose, and xylose were used as carbon source for levan production. Among the simple sugars used lactose, galactose and starch failed to support levan production, as confirmed by TLC analysis. Maximum levan production was noticed in the presence of sucrose as the sole carbon source in comparison with other sugars. Among the substrates sugarcane juice 20 % (v/v) and molasses 20 % (v/v), maximum levan was produced when sugarcane juice (50.25 g/L on wet weight basis and 16.35 g/L on dry weight basis) was used as sole carbon source, which was higher than that of medium with sucrose 50 % (w/v) as sole carbon source (43.00 g/L on wet weight basis and 11.25 g/L on dry weight basis) (Fig. 4). Increased levan production in sugarcane juice could be due to its complex nature with high concentration of sucrose and mineral salts necessary for EPS production [20]. Cane molasses resulted in the production of levan at lower level which could be due to presence of potassium, chloride, sulfur, calcium, and sodium salts [21] or trace metals such as iron, zinc, and copper [22] inhibiting bacterial growth and levan production. Among the organic nitrogen sources tested at 2 % (w/v), casein peptone supported the highest levan production (50.25 g/L on wet weight basis and 16.35 g/L on dry weight basis) when used as the sole nitrogen source in comparison with protease peptone and yeast extract (Fig. 4). These findings are accordance with the findings reported by Nagnath et al. [7] wherein P. fluorescence NCIM 2059 was studied using casein peptone at 10 g/L along with sugarcane juice as sole carbon source yielded levan at 7.8 g/L.
Optimization of Selective Medium Components by Central Composite Design (CCD)
In the present study, the relationship between the response functions and process variables was identified by three-factor inscribed composite design. Production of levan at 35 °C for 48 h under agitation at 200 rpm was determined from previous one-factor-at-a-time experiments. Further, variables such as sugarcane juice as carbon source, casein peptone as nitrogen source, and pH of the media for production of levan by B. licheniformis ANT 179 were optimized by central composite design using RSM. The predicted and experimentally measured responses for 20 runs according to the experimental design are shown in Table 3. Levan yield ranged from 10.21 to 50.25 g/L on wet weight basis, and the maximum yield was obtained for the 20th run under the experimental conditions of X 1 = 20 %, X 2 = 2 %, and X 3 = 7. The lowest yield was obtained for the 17th run with the following conditions of X 1 = 20 %, X 2 = 2 %, and X 3 = 5.36. Based on these data, the media components were optimized for obtaining desirable response, i.e., yield at maximum.
Fitting the Model
The second-order polynomial model equation was obtained by fitting the results obtained as shown in Eq. 3.
Analysis of variance (ANOVA) results indicated that the model is significant (Table 4). The significance of each regression coefficient was determined from the p value of F test (p < 0.05). A lower p value indicates the more significant nature of the corresponding coefficient [23]. Overall, a close relationship between the experimental values and the predicted values indicated that the developed model is satisfactory. The coefficient of regression (R 2) determines the quality of fit of the model, and the R 2 values for levan yield (g/L) were 0.919. The model was confirmed as significant at the level of 0.0001 % probability level with the R 2 > 91.9 % and adjusted R 2 of 84.6 %, where the value of R 2 > 75 % indicates the aptness of the model. This value ensured a satisfactory adjustment of quadratic model to explain the experimental data and indicated that the model could explain 90 % of the variability in the response. The plot of expected versus residual values of levan yield (supplementary information) also proves the significance of the model as the entire points cluster around the diagonal lines.
Effect of Media Components and pH on Levan Yield
Levan production by B. licheniformis ANT 179 was optimized using the medium containing sugarcane juice as the carbon source and casein peptone as the nitrogen source by CCRD. The fermentation conditions of 48 h incubation at 35 °C with agitation at 200 rpm obtained in preliminary experiments were kept uniform during the optimization process. In the present study, the factors influencing the levan yield were determined by the significant coefficient of the second-order polynomial regression equation. The results indicated that the first-order linear effect was significant for sugarcane juice concentration (X 1) and casein peptone (X 2); second-order quadratic effect was significant for sugarcane juice concentration (X 1), casein peptone (X 2), and pH (X 3); and interactive effect was significant only for sugarcane juice concentration (X 1) and casein peptone (X 2) (Table 3). The data indicated that an increase in sugarcane juice concentrations led to enhanced production of levan. A linear increase in levan content was noticed with increasing concentration of sugarcane juice, and a maximum yield of 50.250 g/L on wet weight basis and 16.35 g/L on dry weight basis was obtained in the medium region of sugarcane juice (20 %), and thereafter above this level, it was found to decrease. Ramsay et al. [24] reported levan production on sucrose containing medium by B. licheniformis, where levan was proposed as a potential and selective plugging agent in microbial-assisted enhanced oil recovery from petroleum sludge. The increased levan production in sugarcane juice could be attributed to the high concentration of sucrose and desirable osmolarity of the medium due to presence of a variety of salts necessary for metabolite production.
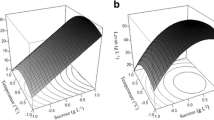
The nitrogen source was also found to be a critical factor determining levan production. An increase in casein peptone concentration resulted in the increase of levan yield. A maximum yield of 50.250 g/L was obtained with casein peptone (2 % (w/v)). It decreased at 3 % (w/v) casein peptone alone. It was also observed that increasing the concentration of casein peptone in combination with sugarcane juice proved to be effective in obtaining higher levan content. The effect of interactive factors of sugarcane juice and casein peptone on levan production is shown in the 3D response surface and the 2D contour plots (Figs. 5 and 6) as a function of sugarcane juice and casein peptone. The 2D contour and 3D response surface plots are the graphical representation of the regression equation. The interaction between the variables can be inferred from the shapes of the surface plots. Kekez et al. [25] reported enhanced production of levan (99.2 g/L) by B. licheniformis NS032 in a response surface methodology optimized medium containing sucrose 397.3 (g/L) and ammonium chloride 4.6 (g/L) as the sole nitrogen source at pH 7.0. Since the pH of the media was a nonsignificant variable, the 3D plot is shown at its zero level of X 3. These data are indicative of significant interaction in the limit of 95 % (p > 0.05), between the concentration of sugarcane juice and casein peptone.
Optimization of Levan Production and Verification of the Model
The numerical optimization of extraction parameters were carried out using Minitab statistical software based on the initial experimental results. Our target was to obtain the highest yield of levan by optimization of carbon and nitrogen components of the fermentation medium. The experiment was conducted according to the predicted and optimal conditions of 20 % (v/v) sugarcane juice concentration and 2 % (w/v) casein peptone. The adequacy of the model in predicting the response efficiently was verified by comparing the observed and predicted value of the responses. The results of the measured responses were in close agreement with predicated value, and the deviation was found to be insignificant (p < 0.05) (Table 5).
Analysis of Characterization of FOS and Levan by TLC and HPLC Analysis
The enzymatic synthesis products of FOS and acid hydrolysis products of levan were analyzed by TLC with fructose as the standard. The enzymatic synthesis of FOS using levansucrase from B. licheniformis ANT 179, for 24 and 48 h, indicated the presence of low molecular weight products, with Rf values matching with fructose, sucrose, and 1-kestose (Fig. 7b). Fructose was also produced as a product during hydrolysis which is due to the exohydrolase property of most reported levansucrases as described in the http://www.brenda-enzymes.info internet page for the EC 3.2.1.65 levanase enzymes. To corroborate this, Tambara et al. [23] also reported 1-kestose as a major transfructosylation product after enzymatic hydrolysis of sucrose using levansucrase from Geothermophilus diazotrophicus. Levan produced from different carbon sources such as sugarcane juice and sucrose was acid hydrolyzed, and the hydrolysis product, i.e., fructose, was compared with the standard (Fig. 7a).
FT-IR and NMR Analysis
The synthesized FOS and levan were subjected to FT-IR analysis. The FT-IR spectra of FOS synthesized using levansucrase produced by 24 and 48 h fermentation of B. licheniformis ANT 179 are shown (Fig. 8a, b). The spectra showed distinct bands in the regions between 1200 and 900 and 3400–2800 cm−1. The sharp peaks observed in the spectral range 1200–900 cm−1 correspond to C–C, C–O stretching, and C–O–H, C–O–C deformation modes of oligosaccharides [26]. A broad stretching peak at around 3332.9 cm−1 (O–H stretching), a weak C–H band at around 2978.0 cm−1, C–O stretching at 1087.8 cm−1, and another sharp peak around 1049 cm−1 are typical of carbohydrates [27]. The infrared spectrum of the levan produced in the study showed the characteristic peak signals of polysaccharides: broad stretching peak at around 3266.7 cm−1 (O–H stretching), a weak C–H band at around 2972.2 cm−1, C–O stretching at 1042.7 cm−1, and several sharp peaks around 1000 cm−1 typical of carbohydrates. The FT-IR of levan obtained showed similarity with that of the commercially available levan, and major peaks associated with produced levan and standard levan are shown in Fig. 9 and supplementary information. FT-IR spectroscopy analysis of levan produced by Halomonas sp. also showed the characteristic peak signals of polysaccharides: broad stretching peak at around 3300 cm−1, due to OH stretching, a weak C–H band at around 2900 cm−1, C–O stretching at 1660 cm−1, and several sharp peaks around 1000 cm−1 [28]. However, non-purified samples were found to contain amino groups as impurities. After purification, all amino groups were removed and FT-IR of the sample showed almost 99 % similarities, confirming that the biopolymer obtained was levan.
FOS used in the present study were the products of enzymatic synthesis from sucrose (Fig. 10). The samples were subjected to 13C NMR analysis and interpreted based on previously available literature. Proton and carbon chemical alignments of three kestoses, namely, 1-kestose [(O-β-d-fructofuranosyl-(2→1)-β-d-fructofuranosyl–(2→1)-α-d-glucopyranoside], 6-kestose [(O-β-d-fructofuranosyl-(2→6)-β-d-fructofuranosyl–(2→1)-α-D-glucopyranoside], and neokestose [(O-β-d-fructofuranosyl-(2→6)-α-d-fructofuranosyl–(1→2)-glucopyranoside] have been reported earlier [29, 30]. The 13C NMR spectrum indicated the presence of unreacted sucrose along with trace amounts of free fructose and glucose as well as kestose and nystose. The occurrence of free glucose in α- and β-forms was observed as carbon signals at 92.2 ppm for α and 95.7 ppm for β. Subsequently, the signals for fructose were observed at 103 ppm which indicated the occurrence of β-linkage. The C-1 and C-6 signals of fructose were also observed in between 60.7 and 61.3 ppm. The chemical shift values of 13C NMR analysis of FOS assigned in the present study were reported earlier [31–33]. Mabel et al. [34] reported that in kestose and nystose type of FOS, the linkage between glucose and fructose is 1→2 and α for the 1-linked glycosidic linkage and β for the 1→2-linked glycosidic linkage of fructose. Thus, the NMR study indicates the occurrence of nystose and kestose in the FOS samples prepared using the levansucrase.
The 13C NMR spectrum of levan polysaccharide produced by B. licheniformis ANT 179 showed the presence of six-well resolved peaks at 62.6, 65.9, 77.8, 78.9, 82.9, and 106.8 ppm (Fig. 11a, b). The carbon chemical shifts are characteristic of β-configured fructofuranose units, as found by comparison with carbon chemical shifts of the standard methyl glycoside [35]. Our studies were in accordance with Poli et al. [28] where 13C NMR spectra of levan produced by Halomonas sp. AAD6 (JCM 15723) strain was composed of repeating units of β-(2,6)-d-fructofuranosyl residues with carbon resonances at 62.5, 65.9, 77.2, 79.8, 82.8, and 106.9. Tomasic et al. [36] also attributed the presence of a downfield shifted signal at 65.9 ppm as the β(2→6) backbone structure of levan-type polysaccharide. Shih et al. [37] and Esawy et al. [38] also confirmed the presence of β-(2,6)-fructofuranoside linkages in levan produced by B. subtilis Natto and B. subtilis M with main carbon resonances at 60.1, 63.6, 75.4, 76.5, 80.5, and 104.4 ppm by 13C NMR analysis.
Conclusions
Levansucrase derived from B. licheniformis ANT 179 could be a useful catalyst for production of FOS as important prebiotic molecules through the process of sucrose hydrolysis. The efficient levan production in an optimized medium with low-cost regional substrates was also carried out which resulted in higher levan production, and our experiments revealed the in vitro production of FOS. Probably the bacterium produces levan to retain moisture under extreme climatic conditions prevailing in the Antarctica. This polymer might be helpful to withstand temperature fluctuations and desiccation during different seasons. B. licheniformis ANT 179 is more useful for levan production for food and biomedical applications as it is a GRAS organism. The prebiotic and immunomodulatory effects of levan have been well established. The optimization of levan production and FOS by the bacterium is a step to further knowledge and application of these molecules.
References
Morianoa, P.S., Lucia, F. A, Ana, P., Jesus, J.B., Antonio, O.B., Francisco, J.P. (2015) Levan versus fructooligosaccharide synthesis using the levansucrase from Zymomonas mobilis: effect of reaction conditions. Journal of Molecular Catalysis B.
Delgado, G. T. C., Wirla, M. S. C. T., & Glaucia, M. P. (2010). Immunomodulatory effects of fructans. Food Research International, 43, 1231–1236.
Lorenzoni, A. S. G., Luiza, F. A., Manuela, P. K., Rafael, C. R., & Plinho, F. H. (2014). Fructooligosaccharides synthesis by highly stable immobilized β-fructofuranosidase from Aspergillus aculeatus. Carbohydrate Polymers, 103, 193–197.
Park, J. P., Tae-Kwang, O., & Jong-Won, Y. (2001). Purification and characterization of a novel transfructosylating enzyme from Bacillus macerans EG-6. Process Biochemistry, 37, 471–476.
Srikanth, R., Gudimalla, S., Chinta, H. S. S., Sundhar, R., Harish, B. S., Janaki, R. M., & Kiran, B. U. (2015). Antioxidant and anti-inflammatory levan produced from Acetobacter xylinum NCIM 2526 and its statistical optimization. Carbohydrate Polymers, 123, 8–16.
Song, E., Hyunjin, K., Sung, H., & Jaeho, C. (2002). Cloning and characterization of a levan biohydrolase from Microbacterium laevaniformans ATCC 15953. Gene, 291, 45–55.
Nagnath, R. J., Mahesh, V. B., Ashwini, V. T., & Uday, S. A. (2012). Microbial Levan from Pseudomonas fluorescens: characterization and medium optimization for enhanced production. Food Science and Biotechnology, 21(4), 1045–1053.
Finore, H., Di Donato, P., Mastascusa, V., Nicolaus, B., & Poli, A. (2014). Fermentation technologies for the optimization of marine microbial exopolysaccharides production. Marine Drugs, 12, 3005–3024.
Antony, R., Krishnana, K. P., Laluraj, C. M., Thambana, M., Dhakephalkar, P. K., Anupama, S. E., & Shivaji, S. (2012). Diversity and physiology of culturable bacteria associated with a coastal Antarctic ice core. Microbiological Research, 167(6), 372–380.
Ramana, K. V., Singh, L., & Nalini, S. (2000). Psychrotrophic hydrolytic bacteria from Antarctica and other low temperature habitats. Defence Science Journal, 2, 177–181.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Senthilkumar, V., & Gunasekaran, P. (2005). Influence of fermentation conditions on levan production by Zymomonas mobilis CT 2. Indian Journal of Biotechnology, 4, 491–496.
Mckenzie, J. D. (2004). Minitab student release 14 statistical software for education. Boston: Pearson Addison-Wesley.
Myers, R. H., & Montgomery, D. C. (2002). Response surface methodology: process and product optimization using designed experiments (2nd ed.). New York: Wiley.
Ammar, Y. B., Matsubara, T., Ito, K., Iizuka, M., Limpaseni, T., Pongsawasdi, P., & Minamiura, N. (2002). Characterization of a thermostable levansucrase from Bacillus sp. TH4-2 capable of producing high molecular weight levan at high temperature. Journal of Biotechnology, 99, 111–119.
Velázquez-Hernández, M. L., Baizabal-Aguirre, V. M., Bravo-Patiño, M. A., Cajero-Juárez, M. P., Chávez-Moctezuma, M. P., & Valdez-Alarcón, J. J. (2009). Microbial fructosyltransferases and the role of fructans. Journal of Applied Microbiology, 106(6), 1763–1778.
Dube, S., Alam, S. I., & Singh, L. (2001). Proteolytic anaerobic bacteria from lake 396 sediments of Antarctica. Enzyme Microbial Technology, 28, 114–121.
Lu, L., Feng, F., Renfei, Z., Lan, J., Chunjuan, H., Li, X., & Min, X. (2014). A recombinant levansucrase from Bacillus licheniformis 8-37-0-1 catalyzes versatile transfructosylation reactions. Process Biochemistry, 49(9), 1503–1510.
Abdel-Fattah, A. F., Mahmoud, D. A. R., & Esawy, M. A. T. (2005). Production of levansucrase from Bacillus subtilis NCR 33 A and enzyme synthesis of levan and fructo-oligosaccharides. Current Microbiology., 55, 402–407.
Ikram-ul, H. A., Sikander, M. A., & Qadeer, I. J. (2002). Citric acid fermentation by mutant strain of Aspergillus niger GCMC-7 using molasses based medium. Electronic Journal of Biotechnology, 5, 125–132.
Paramjit, S. P. (2008). Application of response surface methodology in the permeabilization of yeast cells for lactose hydrolysis. Journal of Biochemical Engineering., 39, 91–96.
de Oliveira, M. R., da Silva, R. S. S. F., Buzato, J. B., & Colabone, C. M. A. P. (2007). Study of levan production by Zymomonas mobilis using regional low-cost carbohydrate sources. Biochemical Engineering Journal, 37, 77–183.
Tambara, Y., Hormaza, J. V., Perez, C., Leon, A., Arrieta, J., & Hernandez, L. (1999). Structural analysis and optimized production of FOS by levan sucrose from Acetobacter diazotrophics SRT4. Biotechnology Letters, 32, 117–121.
Ramsay, A., Cooper, D. G., & Neufeld, R. J. (1989). Effects of oil reservoir conditions on the production of water-insoluble levan by Bacillus licheniformis. Geomicrobiology Journal, 7(3), 155–165.
Kekez, B. D., Gojgic-Cvijovic, G. D., Jakovljevic, D. M., Stefanovic, K. J. R., Markovic, M. D., Beskoski, V. P., & Vrvic, M. M. (2015). High levan production by Bacillus licheniformis NS032 using ammonium chloride as the sole nitrogen source. Applied Biochemistry and Biotechnology, 75(6), 3068–3083.
Grube, M., Bekers, M., Upite, D., & Kaminska, E. (2002). IR-spectroscopic studies of Zymomonas mobilis and levan precipitate. Vibrational Spectroscopy, 28, 277–285.
Bagheri, L., Madadlou, A., Yarmand, M., & Mousavi, M. E. (2013). Nanoencapsulation of date palm pit extract in whey protein particles generated via desolvation method. Food Research International, 51(2), 866–871.
Poli, A., Hande, K., Bahar, G., Giuseppina, T., Giuseppina, P., Toksoy Oner, E., & Barbara, N. (2009). High level synthesis of levan by a novel Halomonas species growing on defined media. Carbohydrate Polymers, 78, 651–657.
Calub, T. M., Waterhouse, A. L., & Chatterton, N. J. (1990). Proton and carbon chemical shift assignments for 1-kestose from two-dimensional NMR spectral measurements. Carbohydrate Research, 199, 11–17.
Liu, J., Waterhouse, A. L., & Chatterton, N. J. (1991). Proton and carbon chemical shift assignments for 6-kestose and neo kestose from two dimensional NMR measurements. Carbohydrate Research, 217, 43–49.
Fujita, K., Kuwahara, N., Tanimoto, T., Koizumi, K., Iizuka, M., Minamiura, N., Furuichi, K., & Kitahata, S. (1994). Chemical structures of heterooligosaccharides produced by Arthrobacter sp. K-1 β-fructofuranosidase. Bioscience Biotechnology and Biochemistry, 58, 239–243.
Barthomeuf, C., Grizard, D., & Teulade, J. C. (1997). Assay and structural determination of fructooligosaccharides synthesized by an enzymatic system from Penicillium rugulosum. Biotechnology Techniques, 11, 845–848.
Hayashi, S., Yoshiyama, T., Fuji, N., & Shinohara, S. (2000). Production of a novel syrup containing neofructooligosaccharides by the cells of Penicillium citrinum. Biotechnology Letters, 22, 1465–1469.
Mabel, M. J., Sangeetha, P. T., Kalpana, P. K., Srinivasan, K., & Prapullaa, S. G. (2008). Physicochemical characterization of fructooligosaccharides and evaluation of their suitability as a potential sweetener for diabetics. Carbohydrate Research, 343, 56–66.
Bock, K., & Pedersen, C. (1983). Carbon-13 nuclear magnetic resonance spectroscopy of monosaccharides. Advances in Carbohydrate Chemistry and Biochemistry, 41, 27–66.
Tomašić, J., Jennings, H. J., & Glaudemans, C. P. J. (1978). Evidence for a single type of linkage in a fructofuranan from Lolium perenne. Carbohydrate Research, 62, 127–133.
Shih, I., Yu, Y., Shieh, C., & Hsieh, C. (2005). Selective production and characterization of levan by Bacillus subtilis (Natto) Takahashi. Journal of Agricultural and Food Chemistry, 53, 8211–8215.
Esawy, M. A., Ahmed, E. F., Wafaa, A. H., Mansour, N. M., El-Senousy, W. M., & El-Safty, M. M. (2012). Antiviral levans from Bacillus spp. isolated from honey. In K. D. Nedra (Ed.), The complex world of polysaccharides, InTech .ISBN 978-953-51-0819-1, chapter 7
Acknowledgments
We thank the Chairperson, Sophisticated Instrument Facility, Indian Institute of Science, Bangalore, India for providing NMR facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xavier, J.R., Ramana, K.V. Optimization of Levan Production by Cold-Active Bacillus licheniformis ANT 179 and Fructooligosaccharide Synthesis by Its Levansucrase. Appl Biochem Biotechnol 181, 986–1006 (2017). https://doi.org/10.1007/s12010-016-2264-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2264-8