Abstract
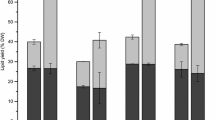
Commercial exploitation of microalgae for biofuel and food ingredients is hindered due to laborious extraction protocols and use of hazardous chemicals. Production of lipids in the microalga grown in modified BG11 medium was evaluated to arrive at the appropriate harvesting conditions. The use of three phase partitioning (TPP) as a green approach for extraction of lipids from Chlorella saccharophila was investigated. Cells disrupted by probe sonication were used for separation of lipids by TPP. The TPP-optimized conditions of 30 % ammonium sulfate, using slurry/t-butanol of 1:0.75 for 60 min at 25 to 35 °C, showed a lipid recovery of 69.05 ± 3.12 % (w/w) as against 100 % (w/w) by using chloroform–methanol extraction. Subsequently, parameters of high-pressure homogenization for cell disruption were optimized for maximum recovery of lipids by TPP. A final recovery of 89.91 ± 3.69 % (w/w) lipids was obtained along with ∼1.26 % w/w carotenoids of dry biomass in the t-butanol layer and protein content of ∼12 % w/w of dry biomass in the middle protein layer due to ammonium sulfate precipitation, after performing TPP under the optimized conditions.

ᅟ




Similar content being viewed by others
References
Lang, T. (2010). Crisis? What crisis? The normality of the current food crisis. Journal of Agrarian Change, 10(1), 87–97. doi:10.1111/j.1471-0366.2009.00250.x.
Araujo, G. S., Matos, L. J. B. L., Fernandes, J. O., Cartaxo, S. J. M., Gonçalves, L. R. B., Fernandes, F. A. N., & Farias, W. R. L. (2013). Extraction of lipids from microalgae by ultrasound application: prospection of the optimal extraction method. Ultrasonics Sonochemistry, 20(1), 95–98. doi:10.1016/j.ultsonch.2012.07.027.
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25(3), 294–306. doi:10.1016/j.biotechadv.2007.02.001.
Ahmad, A. L., Yasin, N. H. M., Derek, C. J. C., & Lim, J. K. (2011). Microalgae as a sustainable energy source for biodiesel production: a review. Renewable and Sustainable Energy Reviews, 15(1), 584–593. doi:10.1016/j.rser.2010.09.018.
MacDougall, K. M., McNichol, J., McGinn, P. J., O’Leary, S. J. B., & Melanson, J. E. (2011). Triacylglycerol profiling of microalgae strains for biofuel feedstock by liquid chromatography–high-resolution mass spectrometry. Analytical and Bioanalytical Chemistry, 401(8), 2609–2616. doi:10.1007/s00216-011-5376-6.
Ranjan, A., Patil, C., & Moholkar, V. S. (2010). Mechanistic assessment of microalgal lipid extraction. Industrial & Engineering Chemistry Research, 49(6), 2979–2985. doi:10.1021/ie9016557.
Guedes, A. C., Amaro, H. M., & Malcata, F. X. (2011). Microalgae as sources of carotenoids. Marine Drugs, 9(12), 625–644. doi:10.3390/md9040625.
Spolaore, P., Joannis-Cassan, C., Duran, E., & Isambert, A. (2006). Commercial applications of microalgae. Journal of Bioscience and Bioengineering, 101(2), 87–96. doi:10.1263/jbb.101.87.
Cheng, C.-H., Du, T.-B., Pi, H.-C., Jang, S.-M., Lin, Y.-H., & Lee, H.-T. (2011). Comparative study of lipid extraction from microalgae by organic solvent and supercritical CO2. Bioresource Technology, 102(21), 10151–10153. doi:10.1016/j.biortech.2011.08.064.
Balasubramanian, R. K., Yen Doan, T. T., & Obbard, J. P. (2013). Factors affecting cellular lipid extraction from marine microalgae. Chemical Engineering Journal, 215–216, 929–936. doi:10.1016/j.cej.2012.11.063.
Kates, M. (1986). Lipid extraction procedures techniques of lipidology: isolation, analysis and identification of lipids. In: R.H. Burdon, P.H. Knippenber, (Eds.), Laboratory techniques in biochemistry and molecular biology (pp. 463–464). Elsevier.
Young, G., Nippgen, F., Titterbrandt, S., & Cooney, M. J. (2010). Lipid extraction from biomass using co-solvent mixtures of ionic liquids and polar covalent molecules. Separation and Purification Technology, 72(1), 118–121. doi:10.1016/j.seppur.2010.01.009.
Alfonsi, K., Colberg, J., Dunn, P. J., Fevig, T., Jennings, S., Johnson, T. A., & Stefaniak, M. (2008). Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation. Green Chemistry, 10(1), 31. doi:10.1039/b711717e.
Mendes, R. L., Reis, A. D., & Palavra, A. F. (2006). Supercritical CO2 extraction of γ-linolenic acid and other lipids from Arthrospira (Spirulina) maxima: comparison with organic solvent extraction. Food Chemistry, 99(1), 57–63. doi:10.1016/j.foodchem.2005.07.019.
Samarasinghe, N., Fernando, S., Lacey, R., & Faulkner, W. B. (2012). Algal cell rupture using high pressure homogenization as a prelude to oil extraction. Renewable Energy, 48, 300–308. doi:10.1016/j.renene.2012.04.039.
Spiden, E. M., Yap, B. H. J., Hill, D. R. A., Kentish, S. E., Scales, P. J., & Martin, G. J. O. (2013). Quantitative evaluation of the ease of rupture of industrially promising microalgae by high pressure homogenization. Bioresource Technology, 140, 165–171. doi:10.1016/j.biortech.2013.04.074.
Dennison, C., & Lovrien, R. (1997). Three phase partitioning: concentration and purification of proteins. Protein Expression and Purification, 11(2), 149–161. doi:10.1006/prep.1997.0779.
Sharma, A., & Gupta, M. N. (2001). Three phase partitioning as a large-scale separation method for purification of a wheat germ bifunctional protease/amylase inhibitor. Process Biochemistry, 37(2), 193–196. doi:10.1016/S0032-9592(01)00199-6.
Rajeeva, S., & Lele, S. S. (2011). Three-phase partitioning for concentration and purification of laccase produced by submerged cultures of Ganoderma sp. WR-1. Biochemical Engineering Journal, 54(2), 103–110. doi:10.1016/j.bej.2011.02.006.
Sharma, A., Khare, S., & Gupta, M. N. (2002). Three phase partitioning for extraction of oil from soybean. Bioresource Technology, 85(3), 327–329. doi:10.1016/S0960-8524(02)00138-4.
Roy, I., Sharma, A., & Gupta, M. N. (2005). Recovery of biological activity in reversibly inactivated proteins by three phase partitioning. Enzyme and Microbial Technology, 37(1), 113–120. doi:10.1016/j.enzmictec.2005.02.007.
Kiss, E., Szamos, J., Tamás, B., & Borbás, R. (1998). Interfacial behavior of proteins in three-phase partitioning using salt-containing water/tert-butanol systems. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 142(2-3), 295–302. doi:10.1016/S0927-7757(98)00361-6.
Chougle, J. A., Singhal, R. S., & Baik, O.-D. (2014). Recovery of astaxanthin from Paracoccus NBRC 101723 using ultrasound-assisted three phase partitioning (UA-TPP). Separation Science and Technology, 49(6), 811–818. doi:10.1080/01496395.2013.872146.
Shah, S., Sharma, A., & Gupta, M. N. (2004). Extraction of oil from Jatropha curcas L. seed kernels by enzyme assisted three phase partitioning. Industrial Crops and Products, 20(3), 275–279. doi:10.1016/j.indcrop.2003.10.010.
Gaur, R., Sharma, A., Khare, S. K., & Gupta, M. N. (2007). A novel process for extraction of edible oils: enzyme assisted three phase partitioning (EATPP). Bioresource Technology, 98(3), 696–699. doi:10.1016/j.biortech.2006.01.023.
Kurmudle, N. N., Bankar, S. B., Bajaj, I. B., Bule, M. V., & Singhal, R. S. (2011). Enzyme-assisted three phase partitioning: a novel approach for extraction of turmeric oleoresin. Process Biochemistry, 46(1), 423–426. doi:10.1016/j.procbio.2010.09.010.
Harde, S. M., & Singhal, R. S. (2012). Extraction of forskolin from Coleus forskohlii roots using three phase partitioning. Separation and Purification Technology, 96, 20–25. doi:10.1016/j.seppur.2012.05.017.
Li, Z., Jiang, F., Li, Y., Zhang, X., & Tan, T. (2013). Simultaneously concentrating and pretreating of microalgae Chlorella spp. by three-phase partitioning. Bioresource Technology, 149, 286–291. doi:10.1016/j.biortech.2013.08.156.
Collos, Y., Mornet, F., Sciandra, A., Waser, N., Larson, A. & Harrison, P. J. An optical method for the rapid measurement. (n.d.).
Jones, J., Manning, S., Montoya, M., Keller, K., & Poenie, M. (2012). Extraction of algal lipids and their analysis by HPLC and mass spectrometry. Journal of the American Oil Chemists’ Society. doi:10.1007/s11746-012-2044-8.
Schoor, A., Erdmann, N., Effmert, U., & Mikkat, S. (1995). Determination of the cyanobacterial osmolyte glucosylglycerol by high-performance liquid chromatography. Journal of Chromatography. A, 704(1), 89–97. doi:10.1016/0021-9673(95)00181-L.
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356. doi:10.1021/ac60111a017.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Wellburn, A. R. (1994). The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology, 144(3), 307–313. doi:10.1016/S0176-1617(11)81192-2.
Jin, H.-F., Lim, B.-R., & Lee, K. (2006). Influence of nitrate feeding on carbon dioxide fixation by microalgae. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 41(12), 2813–2824. doi:10.1080/10934520600967928.
Baldwin, R. L. (1996). How Hofmeister ion interactions affect protein stability. Biophysical Journal, 71(4), 2056–2063. doi:10.1016/S0006-3495(96)79404-3.
Reeve, W., Erikson, C. M., & Aluotto, P. F. (1979). A new method for the determination of the relative acidities of alcohols in alcoholic solutions. The nucleophilicities and competitive reactivities of alkoxides and phenoxides. Canadian Journal of Chemistry, 57(20), 2747–2754. doi:10.1139/v79-444.
Thorne, K. J. I., & Barker, D. C. (1969). Bactoprenol, ATPase and acetate activating enzymes of a vesicular fraction from Lactobacillus casei. European Journal of Biochemistry, 11(3), 582–591. doi:10.1111/j.1432-1033.1969.tb00810.x.
Vidhate, G. S., & Singhal, R. S. (2013). Extraction of cocoa butter alternative from kokum (Garcinia indica) kernel by three phase partitioning. Journal of Food Engineering, 117(4), 464–466. doi:10.1016/j.jfoodeng.2012.10.051.
Chisti, Y., & Moo-Young, M. (1986). Disruption of microbial cells for intracellular products. Enzyme and Microbial Technology, 8(4), 194–204. doi:10.1016/0141-0229(86)90087-6.
Becker, W. (2003). Microalgae in human and animal nutrition. In A. Richmond (Ed.), Handbook of microalgal culture (pp. 312–351). Oxford, UK: Blackwell Publishing Ltd.. Retrieved from http://doi.wiley.com/10.1002/9780470995280.ch18.
Richmond, A. (Ed.). (2004). Handbook of microalgal culture: biotechnology and applied phycology. Oxford: Blackwell Science.
Burczyk, J., Śmietana, B., Termińska-Pabis, K., Zych, M., & Kowalowski, P. (1999). Comparison of nitrogen content amino acid composition and glucosamine content of cell walls of various chlorococcalean algae. Phytochemistry, 51(4), 491–497. doi:10.1016/S0031-9422(99)00063-1.
Singh, D., Puri, M., Wilkens, S., Mathur, A. S., Tuli, D. K., & Barrow, C. J. (2013). Characterization of a new zeaxanthin producing strain of Chlorella saccharophila isolated from New Zealand marine waters. Bioresource Technology, 143, 308–314. doi:10.1016/j.biortech.2013.06.006.
Acknowledgments
The authors would like to thank the Department of Biotechnology, Government of India for their financial support. We thank DBT-ICT-CEB, Mumbai for providing us with C. saccharophila. We also thank Dr. Reena Pandit and Ms. Sujata Gaikwad, DBT-ICT-CEB for their support and valuable inputs in the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mulchandani, K., Kar, J.R. & Singhal, R.S. Extraction of Lipids from Chlorella saccharophila Using High-Pressure Homogenization Followed by Three Phase Partitioning. Appl Biochem Biotechnol 176, 1613–1626 (2015). https://doi.org/10.1007/s12010-015-1665-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1665-4