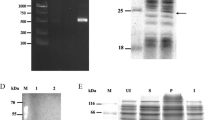
Abstract
Dehydrins are a group of plant proteins that have been shown to be involved in the tolerance of various abiotic stresses such as dehydration, salinity, and low temperature. We have previously shown that the K-segments of the wheat dehydrin DHN-5 are essential for the protection of enzyme activities in vitro. In this study, we further investigate the role of the K-segments in the growth of Escherichia coli under various stresses, and we tested their antibacterial and antifungal activities. Our results showed that the truncated forms of DHN-5 containing the two K-segments enhanced tolerance of E. coli against diverse stresses by protecting proteins against aggregation. In addition, we demonstrated that the K-segments have antibacterial and antifungal activities against Gram-positive and Gram-negative bacteria and fungi. Based on these results, we propose that the K-segments may play a protective role in plants not only under abiotic stress conditions but also most likely during defense mechanisms.





Similar content being viewed by others
References
Dunker, A. K., Obradovic, Z., Romero, P., Garner, E. C., & Brown, C. J. (2000). Intrinsic protein disorder in complete genomes. Genome Inform Ser Workshop Genome Information, 11, 161–171.
Hanin, M., Brini, F., Ebel, C., Toda, Y., Takeda, S., & Masmoudi, K. (2011). Plant dehydrins and stress tolerance: versatile proteins for complex mechanisms. Plant Signaling & Behavior, 6, 1–7.
Kovacs, B., & Tompa, P. (2012). Diverse functional manifestations of intrinsic structural disorder in molecular chaperones. Biochemical Society Transaction, 4, 963–968.
Tompa, P., Szász, C., & Buday, L. (2005). Structural disorder throws new light on moonlighting. Trends in Biochemical Sciences, 30, 484–489.
Battaglia, M., & Covarrubias, A. (2013). Late Embryogenesis Abundant (LEA) proteins in legumes. Frontiers of Plant Science, 4, 190.
Close, T. J. (1996). Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. Plant Physiology, 97, 795–803.
Riera, M., Peracchia, G., de-Nadal, E., Arino, J., & Pagès, M. (2001). Maize protein kinase CK2: regulation and functionality of three regulatory subunits. Plant Journal, 25, 365–374.
Plana, M., Itarte, E., Goday, A., Pagès, M., & Martinez, M. C. (1991). Phosphorylation of maize Rab17 protein by casein kinase 2. Journal of Biological Chemistry, 266, 22510–22514.
Alsheikh, M. K., Heyen, B. J., & Randall, S. K. (2003). Ion binding properties of the dehydrin ERD14 are dependent upon phosphorylation. Journal of Biological Chemistry, 278, 40882–40889.
Goday, A., Jensen, A. B., Culianez-Macia, F. A., Alba, M. M., Figueras, M., Serratosa, J., Torrent, M., & Pages, M. (1994). The maize abscissic acid-responsive protein Rab17 is located in the nucleus and interacts with nuclear localization signals. Plant Cell, 6, 351–360.
Close, T. J., Kortt, A. A., & Chandler, P. M. (1989). A cDNA-based comparison of dehydration-induced proteins (dehydrins) in barley and corn. Plant Molecular Biology, 13, 95–108.
Drira, M., Saibi, W., Brini, F., Gargouri, A., Masmoudi, K., & Hanin, M. (2013). The K-segments of the wheat dehydrin DHN-5 are essential for the protection of lactate dehydrogenase and β-glucosidase activities in vitro. Molecular Biotechnology, 54, 643–650.
Hughes, S., & Graether, S. P. (2011). Cryoprotective mechanism of a small intrinsically disordered dehydrin protein. Protein Science, 20, 42–50.
Rahman, L. N., Chen, L., Nazim, S., Bamm, V. V., Yaish, M. W., Moffatt, B. A., Dutcher, J. R., & Harauz, G. (2010). Interactions of intrinsically disordered Thellungiella salsuginea dehydrins TsDHN-1 and TsDHN-2 with membranes-synergistic effects of lipid composition and temperature on secondary structure. Biochemistry and Cell Biology, 88, 791–807.
Eriksson, S. K, & Harryson, P. (2011). Dehydrins: molecular biology, structure and function. Plant Desiccation Tolerance, 289–305.
Liu, G., Xu, H., Zhang, L., & Zheng, Y. (2011). Fe binding properties of two soybean (Glycine max L.) LEA4 proteins associated with antioxidant activity. Plant Cell Physiology, 52(6), 994–1002.
Chakrabortee, S., Tripathi, R., Watson, M., Schierle, G. S. K., Kurniawan, D. P., Kaminski, C. F., Wise, M. J., & Tunnacliffe, A. (2012). Intrinsically disordered proteins as molecular shields. Molecular Biosystems, 8, 210–219.
Brini, F., Saibi, W., Amara, I., Gargouri, A., Masmoudi, K., & Hanin, M. (2010). The wheat dehydrin DHN-5 exerts a heat protective effect on ß-glucosidase and glucose oxidase activities. Bioscience Biotechnology and Biochemistry, 74, 1050–1054.
Bhardwaj, R., Sharma, I., Kanwar, M., Sharma, R., Handa, N., Kaur, H., Kapoor, D., & Poonam. (2013). LEA proteins in salt stress tolerance. In P. Ahmad, M. M. Azooz, & M. N. V. Prasad (Eds.), Salt stress in plants (pp. 79–112). New York: Springer.
Lan, Y., Cai, D., & Zheng, Y. (2005). Expression in Escherichia coli of three different soybean late embryogenesis abundant (LEA) genes to investigate enhanced stress tolerance. Journal of Integrated Plant Biology, 47, 613–621.
Zhang, L., Ohta, A., Takagi, M., & Imai, R. (2000). Expression of plant group 2 and group 3 LEA genes in Saccharomyces cerevisiae revealed functional divergence among LEA proteins. Journal of Biochemistry (Tokyo), 127, 611–616.
Campos, F., Zamudio, F., & Covarrubias, A. A. (2006). Two different late embryogenesis abundant proteins from Arabidopsis thaliana contain specific domains that inhibit Escherichia coli growth. Biochemical and Biophysical Research Communications, 342, 406–413.
Zhai, C., Lan, J., Wang, H., Li, L., Cheng, X., & Liu, G. (2011). Rice dehydrin Ksegments have in vitro antibacterial activity. Biochemistry (Mosc), 76, 645–650.
Brini, F., Hanin, M., Lumbreras, V., Amara, I., Khoudi, H., Hassairi, A., Pagès, M., & Masamoudi, K. (2007). Overexpression of wheat dehydrin DHN-5 enhances tolerance to salt and osmotic stress in Arabidopsis thaliana. Plant Cell Report, 26, 2017–2026.
Brini, F., Yamamoto, A., Jlaiel, L., Takeda, S., Hobo, T., Dinh, H. Q., Hattori, T., Masmoudi, K., & Hanin, M. (2011). Pleiotropic effects of the wheat dehydrin DHN-5 on stress responses in Arabidopsis. Plant Cell Physiology, 52(4), 676–688.
Gupta, K., Agarwal, P., Reddy, M., & Jha, B. (2010). SbDREB2A, an A-2 type DREB transcription factor from extreme halophyte Salicornia brachiata confers abiotic stress tolerance in Escherichia coli. Plant Cell Report, 29, 1131–1137.
Amara, I., Odena, A., Oliveira, E., Moreno, A., Masmoudi, K., Pagès, M., & Goday, A. (2012). Insights into maize LEA proteins: from proteomics to functional approaches. Plant Cell Physiology, 53, 312–329.
He, S., Tan, L., Hu, Z., Chen, G., Wang, G., & Hu, T. (2012). Molecular characterization and functional analysis by heterologous expression in E. coli under diverse abiotic stresses for OsLEA5, the atypical hydrophobic LEA protein from Oryza sativa L. Molecular Genetics and Genomics, 287, 39–54.
Liu, Y., Zheng, Y., Zhang, Y., Wang, W., & Li, R. (2010). Soybean PM2 protein (LEA3) confers the tolerance of Escherichia coli and stabilization of enzyme activity under diverse stresses. Current Microbiology, 60, 373–378.
Chakrabortee, S., Boschetti, C., Walton, L. J., Sarkar, S., Rubinsztein, D. C., & Tunnacliffe, A. (2007). Hydrophilic protein associated with desiccation tolerance exhibits broad protein stabilization function. Proceedings of the National Academy of Sciences USA, 104, 18073–18078.
Tagg, J. R., & Mcgiven, A. R. (1971). Assay systems for bacteriocins. Applied and Environmental Microbiology, 21, 943–947.
Andrews, J. M. (2001). Determination of minimum inhibitory concentrations. Journal of Antimicrobial Chemotherapy, 48(S1), 5–16.
Pazos, F., Pietrosemoli, N., García-Martín, J. A., & Solano, R. (2013). Protein intrinsic disorder in plants. Frontiers in Plant Science, 4, 363.
Sun, X., Rikkerink, E. H. A., Jones, W. T., & Uversky, V. N. (2013). Multifarious roles of intrinsic disorder in proteins illustrate its broad impact on plant biology. Plant Cell, 25, 38–55.
Dure, L., Crouch, M., Harada, J., Ho, T. H. D., Mundy, J., Quatrano, R., Thomas, T., & Sung, Z. R. (1989). Common amino acids sequence domains among the LEA proteins of higher plants. Plant Molecular Biology, 12, 475–486.
Tunnacliffe, A., & Wise, M. J. (2007). The continuing conundrum of the LEA proteins. Naturwissenschaften, 94, 791–812.
Yang, B., Sugio, A., & White, F. F. (2006). Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proceedings of the National Academy of Sciences USA, 103, 10503–10508.
Koag, M. C., Wilkens, S., Fenton, R. D., Resnik, J., Vo, E., & Close, T. J. (2009). The K-segment of maize DHN1 mediates binding to anionic phospholipid vesicles and concomitant structural changes. Plant Physiology, 150, 1503–1514.
Hughes, S., Schart, V., Malcolmson, J., Hogarth, K., Martynowicz, D. M., Tralman-Baker, E., Patel, S. N., & Graether, S. P. (2013). The importance of size and disorder in the cryoprotective effects of dehydrins. Plant Physiology, 113, 226803.
Pushpanathan, M., Gunasekaran, P., & Rajendhran, J. (2013). Antimicrobial peptides: versatile biological properties. International Journal of Peptides, 2013, 675391.
Acknowledgments
This study was supported by grants from the Ministry of Higher Education and Scientific Research, Tunisia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drira, M., Saibi, W., Amara, I. et al. Wheat Dehydrin K-Segments Ensure Bacterial Stress Tolerance, Antiaggregation and Antimicrobial Effects. Appl Biochem Biotechnol 175, 3310–3321 (2015). https://doi.org/10.1007/s12010-015-1502-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1502-9