Abstract
Background
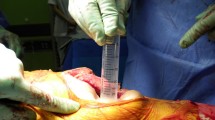
Cyanoacrylate-based, microbial sealant is an adhesive skin barrier designed to prevent bacterial contamination in surgical wounds. This type of adhesive barrier could have use in decreasing the incidence of positive cultures and subsequent infection in shoulder arthroplasty.
Questions/purposes
We therefore evaluated whether cyanoacrylate microbial sealant reduced the positive intraoperative culture rates in revision shoulder arthroplasty.
Methods
We retrospectively reviewed 55 patients who underwent revision shoulder arthroplasties. Intraoperative aerobic and anaerobic deep tissue culture results taken during the revisions were compared. Cultures were taken of the deep synovial tissue lining the prosthesis. Patients were divided into two groups: those who underwent standard preparations with adhesive, iodine-barrier drapes (Group SP) and those who had placement of cyanoacrylate microbial sealant in addition to the standard prep (Group MS).
Results
The prevalence of cases with positive cultures was 18% (seven of 40) in Group SP compared with 7% (one of 15) in Group MS. The prevalence of positive, anaerobic Propionibacterium acnes cultures was 13% in Group SP compared with 7% in Group MS. The prevalence of infections confirmed at revision surgery was 8% in Group SP versus 0% in Group MS.
Conclusions
Our observations suggest application of a cyanoacrylate microbial sealant may reduce the prevalence of positive cultures and thereby subsequent infections in revision shoulder arthroplasties.
Level of Evidence
Level III, retrospective cohort study. See Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
References
Aksoy M, Turnadere E, Ayalp K, Kayabali M, Ertugrul B, Bilgic L. Cyanoacrylate for wound closure in prosthetic vascular graft surgery to prevent infections through contamination. Surg Today. 2006;36:52–56.
Austin L, Zmistowski B, Chang ES, Williams GR Jr. Is reverse shoulder arthroplasty a reasonable alternative for revision arthroplasty? Clin Orthop Relat Res. 2011;469:2531–2537.
Bady S, Wongworawat MD. Effectiveness of antimicrobial incise drapes versus cyanoacrylate barrier preparations for surgical sites. Clin Orthop Relat Res. 2009;467:1674–1677.
Dodson CC, Craig EV, Cordasco FA, Dines DM, Dines JS, Dicarlo E, Brause BD, Warren RF. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elbow Surg. 2010;19:303–307.
Dohmen PM, Gabbieri D, Weymann A, Linneweber J, Geyer T, Konertz W. A retrospective non-randomized study on the impact of INTEGUSEAL, a preoperative microbial skin sealant, on the rate of surgical site infections after cardiac surgery. Int J Infect Dis. 2011;15:e395–400.
Dromzee E, Tribot-Laspiere Q, Bachy M, Zakine S, Mary P, Vialle R. Efficacy of InteguSeal for surgical skin preparation in children and adolescents undergoing scoliosis correction. Spine (Phila Pa 1976). 2012;37:E1331–1335.
Flury MP, Frey P, Goldhahn J, Schwyzer HK, Simmen BR. Reverse shoulder arthroplasty as a salvage procedure for failed conventional shoulder replacement due to cuff failure—midterm results. Int Orthop. 2011;35:53–60.
Foruria AM, Fox TJ, Sperling JW, Cofield RH. Clinical meaning of unexpected positive cultures (UPC) in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2012 Sep 13 [Epub ahead of print].
Grosso MJ, Sabesan VJ, Ho JC, Ricchetti ET, Iannotti JP. Reinfection rates after 1-stage revision shoulder arthroplasty for patients with unexpected positive intraoperative cultures. J Shoulder Elbow Surg. 2012;21:754–758.
Herrera MF, Bauer G, Reynolds F, Wilk RM, Bigliani LU, Levine WN. Infection after mini-open rotator cuff repair. J Shoulder Elbow Surg. 2002;11:605–608.
Iyer A, Gilfillan I, Thakur S, Sharma S. Reduction of surgical site infection using a microbial sealant: a randomized trial. J Thorac Cardiovasc Surg. 2011;142:438–442.
Kelly JD 2nd, Hobgood ER. Positive culture rate in revision shoulder arthroplasty. Clin Orthop Relat Res. 2009;467:2343–2348.
Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89:189–195.
Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994.
Patel A, Calfee RP, Plante M, Fischer SA, Green A. Propionibacterium acnes colonization of the human shoulder. J Shoulder Elbow Surg. 2009;18:897–902.
Pottinger P, Butler-Wu S, Neradilek MB, Merritt A, Bertelsen A, Jette JL, Warme WJ, Matsen FA. Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Joint Surg Am. 2012;94:2075–2083.
Sperling JW, Cofield RH. Revision total shoulder arthroplasty for the treatment of glenoid arthrosis. J Bone Joint Surg Am. 1998;80:860–867.
Surgical Care Improvement Project (SCIP) Measures. Available at: http://www.jointcommission.org/surgical_care_improvement_project. Accessed December 10, 2012.
Topolski MS, Chin PY, Sperling JW, Cofield RH. Revision shoulder arthroplasty with positive intraoperative cultures: the value of preoperative studies and intraoperative histology. J Shoulder Elbow Surg. 2006;15:402–406.
Towfigh S, Cheadle WG, Lowry SF, Malangoni MA, Wilson SE. Significant reduction in incidence of wound contamination by skin flora through use of microbial sealant. Arch Surg. 2008;143:885–891.
Trappey GJ, O’Connor DP, Edwards TB. What are the instability and infection rates after reverse shoulder arthroplasty? Clin Orthop Relat Res. 2011;469:2505–2511.
Von Eckardstein AS, Lim CH, Dohmen PM, Pego-Fernandes PM, Cooper WA, Oslund SG, Kelley EL. A randomized trial of a skin sealant to reduce the risk of incision contamination in cardiac surgery. Ann Thorac Surg. 2011;92:632–637.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained when required.
About this article
Cite this article
Lorenzetti, A.J., Wongworawat, M.D., Jobe, C.M. et al. Cyanoacrylate Microbial Sealant May Reduce the Prevalence of Positive Cultures in Revision Shoulder Arthroplasty. Clin Orthop Relat Res 471, 3225–3229 (2013). https://doi.org/10.1007/s11999-013-2854-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-013-2854-5