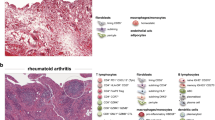
Abstract
Rheumatoid arthritis is characterized by multiple pathobiological processes and heterogeneous clinical phenotypes. Not surprisingly, the inflamed synovium harbors an equally complex pathology. This includes variability in infiltrating and resident cell populations, spatial arrangements, and cell–cell interactions, as well as gene expression profiles. Remarkable progress in our understanding of the many facets of tissue heterogeneity has been facilitated by the increasing availability of patients’ material and the development of advanced research technologies. The next challenge is to capitalize on the large amount of data generated to elucidate the specific pathogenic pathways disparately activated in different patients and/or different phases of the disease. When tissue pathology can be reliably explored through noninvasive circulating biomarkers, then the circle will be closed. We attempt to highlight key advances in the understanding of synovial tissue heterogeneity in rheumatoid arthritis and summarize novel perspectives in synovial biomarker discovery in relation to peripheral blood.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8.
Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–50.
Haldorsen K, Moen K, Jacobsen H, Jonsson R, Brun JG. Exocrine function in primary Sjögren syndrome: natural course and prognostic factors. Ann Rheum Dis. 2008;67:949–54.
Austin HA, Boumpas DT, Vaughan EM, Balow JE. High-risk features of lupus nephritis: importance of race and clinical and histologic factors in 166 patients. Nephrol Dial Transplant. 1995;10:1620–8.
Mittal B, Rennke H, Singh AK. The role of kidney biopsy in the management of lupus nephritis. Curr Opin Nephrol Hypertens. 2005;14:1–8.
Baeten D, Demetter P, Cuvelier C, et al. Comparative study of the synovial histology in rheumatoid arthritis, spondyloarthropathy, and osteoarthritis: influence of disease duration and activity. Ann Rheum Dis. 2000;59:945–53.
van de Sande MG, Thurlings RM, Boumans MJ, et al. Presence of lymphocyte aggregates in the synovium of patients with early arthritis in relationship to diagnosis and outcome: is it a constant feature over time? Ann Rheum Dis. 2010. doi:10.1136/ard.2010.139287.
Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:115–24.
Kraan MC, Haringman JJ, Weedon H, et al. T cells, fibroblast-like synoviocytes, and granzyme B + cytotoxic cells are associated with joint damage in patients with recent onset rheumatoid arthritis. Ann Rheum Dis. 2004;63:483–8.
Tak PP, Taylor PC, Breedveld FC, Smeets TJ, Daha MR, Kluin PM, et al. Decrease in cellularity and expression of adhesion molecules by anti-tumor necrosis factor alpha monoclonal antibody treatment in patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1077–81.
Barrera P, Joosten LA, den Broeder AA, van de Putte LB, van Riel PL, van den Berg WB. Effects of treatment with a fully human anti-tumour necrosis factor alpha monoclonal antibody on the local and systemic homeostasis of interleukin 1 and TNFalpha in patients with rheumatoid arthritis. Ann Rheum Dis. 2001;60:660–9.
Thurlings RM, Vos K, Wijbrandts CA, Zwinderman AH, Gerlag DM, Tak PP. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response. Ann Rheum Dis. 2008;67:917–25.
Kavanaugh A, Rosengren S, Lee SJ, et al. Assessment of rituximab’s immunomodulatory synovial effects (ARISE trial). 1: clinical and synovial biomarker results. Ann Rheum Dis. 2008;67:402–8.
Manzo A, Bombardieri M, Humby F, Pitzalis C. Secondary and ectopic lymphoid tissue responses in rheumatoid arthritis: from inflammation to autoimmunity and tissue damage/remodeling. Immunol Rev. 2010;233:267–85.
Schett G, Firestein GS. Mr Outside and Mr Inside: classic and alternative views on the pathogenesis of rheumatoid arthritis. Ann Rheum Dis. 2010;69:787–9.
van de Sande MG, Gerlag DM, Lodde BM, et al. Evaluating antirheumatic treatments using synovial biopsy: a recommendation for standardisation to be used in clinical trials. Ann Rheum Dis. 2011;70:423–7.
Kraan MC, Reece RJ, Smeets TJ, Veale DJ, Emery P, Tak PP. Comparison of synovial tissues from the knee joints and the small joints of rheumatoid arthritis patients: implications for pathogenesis and evaluation of treatment. Arthritis Rheum. 2002;46:2034–8.
Scirè CA, Epis O, Codullo V, et al. Immunohistological assessment of the synovial tissue in small joints in rheumatoid arthritis: validation of a minimally invasive ultrasound-guided synovial biopsy procedure. Arthritis Res Ther. 2007;9:R101.
Kraan MC, Versendaal H, Jonker M, et al. Asymptomatic synovitis precedes clinically manifest arthritis. Arthritis Rheum. 1998;41:1481–8.
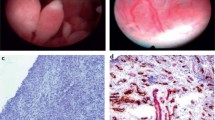
• van de Sande MG, de Hair MJ, van der Leij C, et al: Different stages of rheumatoid arthritis: features of the synovium in the preclinical phase. Ann Rheum Dis 2010, doi:10.1136/ard.2010.139527. This work is the first to investigate the pathological features of the synovial membrane in nonarthritic individuals at risk of developing RA. Availability of synovial tissue from unaffected joints is allowed by mini-invasive small-bore arthroscopy of the knee.
Rooney T, Bresnihan B, Andersson U, et al. Microscopic measurement of inflammation in synovial tissue: inter-observer agreement for manual quantitative, semiquantitative and computerised digital image analysis. Ann Rheum Dis. 2007;66:1656–60.
van der Pouw Kraan TC, van Gaalen FA, Huizinga TW, et al. Discovery of distinctive gene expression profiles in rheumatoid synovium using cDNA microarray technology: evidence for the existence of multiple pathways of tissue destruction and repair. Genes Immun. 2003;4:187–96.
Lindberg J, af Klint E, Ulfgren AK, et al. Variability in synovial inflammation in rheumatoid arthritis investigated by microarray technology. Arthritis Res Ther. 2006;8:R47.
Tak PP, Smeets TJ, Daha MR, et al. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997;40:217–25.
Tsubaki T, Arita N, Kawakami T, et al. Characterization of histopathology and gene-expression profiles of synovitis in early rheumatoid arthritis using targeted biopsy specimens. Arthritis Res Ther. 2005;7:R825–836.
van Oosterhout M, Bajema I, Levarht EW, Toes RE, Huizinga TW, van Laar JM. Differences in synovial tissue infiltrates between anti-cyclic citrullinated peptide-positive rheumatoid arthritis and anti-cyclic citrullinated peptide-negative rheumatoid arthritis. Arthritis Rheum. 2008;58:53–60.
Baeten D, Kruithof E, De Rycke L, et al. Infiltration of the synovial membrane with macrophage subsets and polymorphonuclear cells reflects global disease activity in spondyloarthropathy. Arthritis Res Ther. 2005;7:R359–369.
Haringman JJ, Gerlag DM, Zwinderman AH, et al. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64:834–8.
Wijbrandts CA, Vergunst CE, Haringman JJ, Gerlag DM, Smeets TJ, Tak PP. Absence of changes in the number of synovial sublining macrophages after ineffective treatment for rheumatoid arthritis: implications for use of synovial sublining macrophages as a biomarker. Arthritis Rheum. 2007;56:3869–71.
Baeten D, Houbiers J, Kruithof E, et al. Synovial inflammation does not change in the absence of effective treatment: implications for the use of synovial histopathology as biomarker in early phase clinical trials in rheumatoid arthritis. Ann Rheum Dis. 2006;65:990–7.
David JP, Schett G. TNF and bone. Curr Dir Autoimmun. 2010;11:135–44.
Danks L, Sabokbar A, Gundle R, Athanasou NA. Synovial macrophage-osteoclast differentiation in inflammatory arthritis. Ann Rheum Dis. 2002;61:916–21.
Kasperkovitz PV, Timmer TC, Smeets TJ, et al. Fibroblast-like synoviocytes derived from patients with rheumatoid arthritis show the imprint of synovial tissue heterogeneity: evidence of a link between an increased myofibroblast-like phenotype and high-inflammation synovitis. Arthritis Rheum. 2005;52:430–41.
Bauer S, Jendro MC, Wadle A, et al. Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res Ther. 2006;8:R171.
Tolboom TC, van der Helm-Van Mil AH, Nelissen RG, Breedveld FC, Toes RE, Huizinga TW. Invasiveness of fibroblast-like synoviocytes is an individual patient characteristic associated with the rate of joint destruction in patients with rheumatoid arthritis. Arthritis Rheum. 2005;52:1999–2002.
Holt AP, Haughton EL, lalor PF, Filer A, Buckley CD, Adams DH. Liver myofibroblasts regulate infiltration and positioning of lymphocytes in human liver. Gastroenterology. 2009;136:705–14.
Manzo A, Bugatti S, Caporali R, et al. CCL21 expression pattern of human secondary lymphoid organ stroma is conserved in inflammatory lesions with lymphoid neogenesis. Am J Pathol. 2007;171:1549–62.
Bradfield PF, Amft N, Vernon-Wilson E, et al. Rheumatoid fibroblast-like synoviocytes overexpress the chemokine stromal cell-derived factor 1 (CXCL12), which supports distinct patterns and rates of CD4+ and CD8+ T cell migration within synovial tissue. Arthritis Rheum. 2003;48:2472–82.
Timmer TC, Baltus B, Vondenhoff M, et al. Inflammation and ectopic lymphoid strucutes in rheumatoid arthritis synovial tissues dissected by genomics technology: identification of the interleukin-7 signaling pathway in tissues with lymphoid neogenesis. Arthritis Rheum. 2007;56:2492–502.
Flavell SJ, Hou TZ, Lax S, Filer AD, Salmon M, Buckley CD. Fibroblasts as novel therapeutic targets in chronic inflammation. Br J Pharmacol. 2008;153(1):S241–6.
Takemura S, Braun A, Crowson C, et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167:1072–80.
Manzo A, Paoletti S, Carulli M, et al. Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol. 2005;35:1347–59.
Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T cell activation in rheumatoid synovium is B cell dependent. J Immunol. 2001;167:4710–8.
Yanni G, Whelan A, Feighery C, et al. Contrasting levels of in vitro cytokine production by rheumatoid synovial tissues demonstrating different patterns of mononuclear cell infiltration. Clin Exp Immunol. 1993;93:387–95.
Klimiuk PA, Goronzy JJ, Björnsson J, Beckenbaugh RD, Weyand CM. Tissue cytokine patterns distinguish variants of rheumatoid synovitis. Am J Pathol. 1997;151:1311–9.
Bugatti S, Manzo A, Vitolo B, et al. Clinical, radiographic and biomolecular features of B cell synovitis in rheumatoid arthritis. Arthritis Rheum. 2010;62:S260 [abstract].
•• Humby F, Bombardieri M, Manzo A, et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009;6:e1. The authors took advantage of a combined in vivo and ex vivo approach to demonstrate the functionality of synovial B-cell follicles in RA. They showed that B cells in the context of large lymphoid aggregates express AID, the key enzyme required for somatic hypermutation and class-switch recombination of the immunoglobulin genes, and support the production of ACPA antibodies when xenotransplanted into SCID mice.
•• Scheel T, Gursche A, zacher J, Häupl T, Berek C. V-region gene analysis of locally defined synovial B and plasma cells reveals selected B cell expansion with accumulation of plasma cell clones in rheumatoid arthritis. Arthritis Rheum. 2011;63:63–72. This work aimed to identify the clonality of B cells and plasma cells within RA synovial tissues. Through laser capture microdissection of B cells and plasma cells and Ig VH gene sequencing, the authors elegantly demonstrated that plasma cells are clonally related to B cells, thus providing evidence of the local differentiation of B cells.
Kraan MC, Haringman JJ, Post WJ, Versendaal J, Breedveld FC, Tak PP. Immunohistological analysis of synovial tissue for differential diagnosis in early arthritis. Rheumatology. 1999;38:1074–80.
Klimiuk PA, Sierakowski S, Latosiewicz R, et al. Circulating tumor necrosis factor alpha and soluble tumor necrosis factor receptors in patients with different patterns of rheumatoid synovitis. Ann Rheum Dis. 2003;62:472–5.
Thurlings RM, Wijbrandts CA, Mebius RE, et al. Synovial lymphoid neogenesis does not define a specific clinical rheumatoid arthritis phenotype. Arthritis Rheum. 2008;58:1582–9.
Caňete JD, Celis R, Moll C, et al. Clinical significance of synovial lymphoid neogenesis and its reversal after anti-tumor necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis. 2009;68:751–6.
Klaasen R, Thurlings RM, Wijbrandts CA, et al. The relationship between synovial lymphocyte aggregates and the clinical response to infliximab in rheumatoid arthritis: a prospective study. Arthritis Rheum. 2009;60:3217–4.
Magliozzi R, Howell OW, Reeves C, et al. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010;68:477–93.
Walsh NC, Gravallese EM. Bone remodeling in rheumatic disease: a question of balance. Immunol Rev. 2010;233:301–12.
Crotti TN, Smith MD, Weedon H, et al. Receptor activator NF-kappaB ligand (RANKL) expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathy, osteoarthritis, and from normal patients: semiquantitative and quantitative analysis. Ann Rheum Dis. 2002;61:1047–54.
Haynes DR, Barg E, Crotti TN, et al. Osteoprotegerin expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathies and osteoarthritis and normal controls. Rheumatology. 2003;42:123–34.
Thaunat O, Patey N, Caligiuri G, et al. Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J Immunol. 2010;185:717–28.
Makrygiannakis D, Hermansson M, Ulfgren AK, Nicholas AP, Zendman AJ, Eklund A, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis. 2008;67:1488–92.
Klareskog L, Malmström V, Lundberg K, Padyukov L, Alfredsson L. Smoking, citrullination and genetic variability in the immunopathogenesis of rheumatoid arthritis. Semin Immunol. 2011. doi:10.1016/j.smim.2011.01.014.
Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–6.
Rooney T, Roux-Lombard P, Veale DJ, FitzGerald O, Dayer JM, Bresnihan B. Synovial tissue and serum biomarkers of disease activity, therapeutic response and radiographic progression: analysis of a proof-of-concept randomised clinical trial of cytokine blockade. Ann Rheum Dis. 2010;69:706–14.
•• van Baarsen LG, Wijbrandts CA, Timmer TC, van der Pouw Kraan TC, Tak PP, Verweij CL. Synovial tissue heterogeneity in rheumatoid arthritis in relation to disease activity and biomarkers in peripheral blood. Arthritis Rheum. 2010;62:1602–7. This important work opens the avenue for comparative synovial–peripheral blood profiling in RA. The authors analyzed extensive gene expression of paired synovial tissue biopsies and whole peripheral blood using complementary DNA microarrays. Although the work failed to highlight significant overlap, it provides the rationale for further studies focused on specific peripheral blood cell populations or molecular pathways.
McKinney EF, Lyons PA, Carr EJ, Hollis JL, Jayne DR, Willcocks LC, et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat Med. 2010;16:586–91.
Manzo A, Vitolo B, Humby F, et al. Mature antigen-experienced T helper cells synthesize and secrete the B cell chemoattractant CXCL13 in the inflammatory environment of the rheumatoid joint. Arthritis Rheum. 2008;58:3377–87.
Rioja I, Hughes FJ, Sharp CH, Warnock LC, Montgomery DS, Akil M, et al. Potential novel biomarkers of disease activity in rheumatoid arthritis patients: CXCL13, CCL23, transforming growth factor alpha, tumor necrosis factor receptor superfamily member 9, and macrophage colony-stimulating factor. Arthritis Rheum. 2008;58:2257–67.
Mao Y, Wang M, Zhou Q, et al. CXCL10 and CXCL13 expression were highly up-regulated in peripheral blood mononuclear cells in acute rejection and poor response to anti-rejection therapy. J Clin Immunol. 2010. doi:10.1007/s10875-010-9500-8.
Mao YY, Yang H, Wang M, et al. Feasibility of diagnosing renal allograft dysfunction by oligonucleotide array: gene expression profile correlates with histopathology. Transpl Immunol. 2011;24:172–80.
Rosengren S, Wei N, Kalunian KC, Kavanaugh A, Boyle DL. CXCL13: a novel biomarker of B-cell return following rituximab treatment and synovitis in patients with rheumatoid arthritis. Rheumatology. 2011;50:603–10.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bugatti, S., Manzo, A., Bombardieri, M. et al. Synovial Tissue Heterogeneity and Peripheral Blood Biomarkers. Curr Rheumatol Rep 13, 440–448 (2011). https://doi.org/10.1007/s11926-011-0201-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-011-0201-y