Abstract
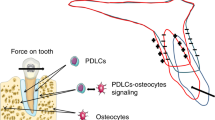
The tooth-periodontal ligament-alveolar bone complex acts symbiotically to dissipate the mechanical loads incurred during mastication and/or orthodontic tooth movement. The periodontal ligament functions both in the tension and compression. At the molecular and celleular levels, the loads in the periodontal ligament trigger mechanobiological events in the alveolar bone, which leads to bone modeling and remodeling. The current review focuses on the bone response to mechanical loading of the periodontal ligament on the tension and pressure sides. Understanding the bone response has major implications for dentistry, including a better understanding of the different types of orthodontic tooth movement.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Reitan K. Tissue behavior during orthodontic tooth movement. Am J Orthod. 1960;46(12):881–900.
Reitan K. Clinical and histologic observations on tooth movement during and after orthodontic treatment. Am J Orthod. 1967;53(10):721–45.
Von Bohl M, Maltha J, Von den Hoff H, Kuijpers-Jagtman AM. Changes in the periodontal ligament after experimental tooth movement using high and low continuous forces in beagle dogs. Angle Orthod. 2004;74(1):16–25.
Meikle MC. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod. 2006;28(3):221–40.
King GJ, Keeling SD, Wronski TJ. Histomorphometric study of alveolar bone turnover in orthodontic tooth movement. Bone. 1991;12(6):401–9.
Leiker BJ, Nanda RS, Currier GF, Howes RI, Sinha PK. The effects of exogenous prostaglandins on orthodontic tooth movement in rats. Am J Orthod Dentofac Orthop. 1995;108(4):380–8.
Masella RS, Meister M. Current concepts in the biology of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2006;129(4):458–68.
Shetty N, Patil AK, Ganeshkar SV, Hegde S. Comparison of the effects of ibuprofen and acetaminophen on PGE2 levels in the GCF during orthodontic tooth movement: a human study. Prog Orthod. 2013;14:6.
Yamasaki K, Miura F, Suda T. Prostaglandin as a mediator of bone resorption induced by experimental tooth movement in rats. J Dent Res. 1980;59(10):1635–42.
Ren Y, Vissink A. Cytokines in crevicular fluid and orthodontic tooth movement. Eur J Oral Sci. 2008;116(2):89–97.
Kapoor P, Kharbanda OP, Monga N, Miglani R, Kapila S. Effect of orthodontic forces on cytokine and receptor levels in gingival crevicular fluid: a systematic review. Prog Orthod. 2014;15:65.
Grieve 3rd WG, Johnson GK, Moore RN, Reinhardt RA, DuBois LM. Prostaglandin E (PGE) and interleukin-1 beta (IL-1 beta) levels in gingival crevicular fluid during human orthodontic tooth movement. Am J Orthod Dentofac Orthop. 1994;105(4):369–74.
Kaku M, Motokawa M, Tohma Y, Tsuka N, Koseki H, Sunagawa H, et al. VEGF and M-CSF levels in periodontal tissue during tooth movement. Biomed Res (Tokyo, Japan). 2008;29(4):181–7.
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–76.
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95(7):3597–602.
Yamaguchi M. RANK/RANKL/OPG during orthodontic tooth movement. Orthod Craniofac Res. 2009;12(2):113–9.
Brooks PJ, Heckler AF, Wei K, Gong SG. M-CSF accelerates orthodontic tooth movement by targeting preosteoclasts in mice. Angle Orthod. 2011;81(2):277–83.
Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27–37.
Uematsu S, Mogi M, Deguchi T. Interleukin (IL)-1 beta, IL-6, tumor necrosis factor-alpha, epidermal growth factor, and beta 2-microglobulin levels are elevated in gingival crevicular fluid during human orthodontic tooth movement. J Dent Res. 1996;75(1):562–7.
van Gastel J, Teughels W, Quirynen M, Struyf S, Van Damme J, Coucke W, et al. Longitudinal changes in gingival crevicular fluid after placement of fixed orthodontic appliances. Am J Orthod Dentofac Orthop. 2011;139(6):735–44.
Khan UA, Hashimi SM, Bakr MM, Forwood MR, Morrison NA. CCL2 and CCR2 are Essential for the Formation of Osteoclasts and Foreign Body Giant Cells. J Cell Biochem. 2016;117(2):382–9.
Wintges K, Beil FT, Albers J, Jeschke A, Schweizer M, Claass B, et al. Impaired bone formation and increased osteoclastogenesis in mice lacking chemokine (C-C motif) ligand 5 (Ccl5). J Bone Miner Res. 2013;28(10):2070–80.
Yu X, Huang Y, Collin-Osdoby P, Osdoby P. CCR1 chemokines promote the chemotactic recruitment, RANKL development, and motility of osteoclasts and are induced by inflammatory cytokines in osteoblasts. J Bone Miner Res Off J Am Soc Bone Miner Res. 2004;19(12):2065–77.
Lean JM, Murphy C, Fuller K, Chambers TJ. CCL9/MIP-1gamma and its receptor CCR1 are the major chemokine ligand/receptor species expressed by osteoclasts. J Cell Biochem. 2002;87(4):386–93.
Taddei SR, Andrade Jr I, Queiroz-Junior CM, Garlet TP, Garlet GP, Cunha Fde Q, et al. Role of CCR2 in orthodontic tooth movement. Am J Orthod Dentofac Orthop. 2012;141(2):153–60.
Teixeira CC, Khoo E, Tran J, Chartres I, Liu Y, Thant LM, et al. Cytokine expression and accelerated tooth movement. J Dent Res. 2010;89(10):1135–41.
Andrade Jr I, Taddei SR, Garlet GP, Garlet TP, Teixeira AL, Silva TA, et al. CCR5 down-regulates osteoclast function in orthodontic tooth movement. J Dent Res. 2009;88(11):1037–41.
Bien SM. Fluid dynamic mechanisms which regulate tooth movement. Adv Oral Biol. 1966;2:173–201.
Bergomi M, Wiskott HW, Botsis J, Mellal A, Belser UC. Load response of periodontal ligament: assessment of fluid flow, compressibility, and effect of pore pressure. J Biomech Eng. 2010;132(1):014504.
Noble BS, Reeve J. Osteocyte function, osteocyte death and bone fracture resistance. Mol Cell Endocrinol. 2000;159(1–2):7–13.
Bonewald LF. The amazing osteocyte. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(2):229–38. This review article focusses on the role of osteocytes on mechanotransduction and activation of osteoclasts.
Matsumoto T, Iimura T, Ogura K, Moriyama K, Yamaguchi A. The Role of Osteocytes in Bone Resorption during Orthodontic Tooth Movement. J Dent Res. 2013;92(4):340–5. The authors showed that the ablation of osteocytes using diptherial toxin in transgenic mice leads to decreased orthodontic tooth movement. The decreased alveolar bone resorption demonstrate the role of osteocytes in the activation of osteoclasts.
Cheung WY, Simmons CA, You L. Osteocyte apoptosis regulates osteoclast precursor adhesion via osteocytic IL-6 secretion and endothelial ICAM-1 expression. Bone. 2012;50(1):104–10.
Al-Dujaili SA, Lau E, Al-Dujaili H, Tsang K, Guenther A, You L. Apoptotic osteocytes regulate osteoclast precursor recruitment and differentiation in vitro. J Cell Biochem. 2011;112(9):2412–23.
Moin S, Kalajzic Z, Utreja A, Nihara J, Wadhwa S, Uribe F, et al. Osteocyte death during orthodontic tooth movement in mice. Angle Orthod. 2014;84(6):1086–92.
Nguyen AM, Jacobs CR. Emerging role of primary cilia as mechanosensors in osteocytes. Bone. 2013;54(2):196–204.
Shalish M, Will LA, Fukai N, Hou B, Olsen BR. Role of polycystin-1 in bone remodeling: orthodontic tooth movement study in mutant mice. Angle Orthod. 2014;84(5):885–90.
Miyagawa A, Chiba M, Hayashi H, Igarashi K. Compressive force induces VEGF production in periodontal tissues. J Dent Res. 2009;88(8):752–6.
Cardaropoli D, Gaveglio L. The Influence of Orthodontic Movement on Periodontal Tissues Level. Semin Orthod. 2007;13(4):234–45.
Norevall LI, Forsgren S, Matsson L. Expression of neuropeptides (CGRP, substance P) during and after orthodontic tooth movement in the rat. Eur J Orthod. 1995;17(4):311–25.
Garlet TP, Coelho U, Silva JS, Garlet GP. Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur J Oral Sci. 2007;115(5):355–62.
Andrade Jr I, Taddei SRA, Souza PEA. Inflammation and Tooth Movement: The Role of Cytokines, Chemokines, and Growth Factors. Semin Orthod. 2012;18(4):257–69.
Pavlin D, Zadro R, Gluhak-Heinrich J. Temporal pattern of stimulation of osteoblast-associated genes during mechanically-induced osteogenesis in vivo: early responses of osteocalcin and type I collagen. Connect Tissue Res. 2001;42(2):135–48.
Pavlin D, Gluhak-Heinrich J. Effect of mechanical loading on periodontal cells. Crit Rev Oral Biol Med. 2001;12(5):414–24.
Gluhak-Heinrich J, Ye L, Bonewald LF, Feng JQ, MacDougall M, Harris SE, et al. Mechanical Loading Stimulates Dentin Matrix Protein 1 (DMP1) Expression in Osteocytes In Vivo. J Bone Miner Res. 2003;18(5):807–17.
Gluhak-Heinrich J, Pavlin D, Yang W, MacDougall M, Harris SE. MEPE expression in osteocytes during orthodontic tooth movement. Arch Oral Biol. 2007;52(7):684–90.
Verna C, Zaffe D, Siciliani G. Histomorphometric study of bone reactions during orthodontic tooth movement in rats. Bone. 1999;24(4):371–9.
Deguchi T, Takano-Yamamoto T, Yabuuchi T, Ando R, Roberts WE, Garetto LP. Histomorphometric evaluation of alveolar bone turnover between the maxilla and the mandible during experimental tooth movement in dogs. Am J Orthod Dentofac Orthop. 2008;133(6):889–97.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sumit Yadav, Ravindra Nanda, and Eliane Dutra declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Craniofacial Skeleton
Rights and permissions
About this article
Cite this article
Dutra, E.H., Nanda, R. & Yadav, S. Bone Response of Loaded Periodontal Ligament. Curr Osteoporos Rep 14, 280–283 (2016). https://doi.org/10.1007/s11914-016-0328-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-016-0328-x