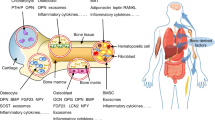
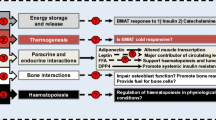
Abstract
Obesity, diabetes, and osteoporosis are major public health concerns. Current estimates indicate that the US population consists of 25% obese, 30% diabetic and prediabetic, and, among the elderly, 50% of all osteoporotic individuals. Mechanistically, these pathologies share several features including common regulators of bone homeostasis and energy metabolism. Peroxisome proliferator-activated receptors (PPARs) represent a family of proteins that control energy turnover in adipose, liver, and muscle tissue. These proteins also control bone turnover and regulate bone cell differentiation. Recent evidence suggests that bone is an organ integral to energy metabolism not only with respect to energy storage, but also as an organ regulating systemic energy homeostasis. In this article, we review current knowledge on the role of PPARs in bone metabolism and bone cell differentiation. We also discuss the role of bone fat in modulation of bone marrow microenvironment and its possible contribution to the systemic regulation of energy metabolism.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
• Lee NK, Sowa H, Hinoi E, et al.: Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130:456–469. This study demonstrates for the first time that bone-derived osteocalcin hormone regulates energy metabolism.
Hinoi E, Gao N, Jung DY, et al.: An osteoblast-dependent mechanism contributes to the leptin regulation of insulin secretion. Ann N Y Acad Sci 2009, 1173(Suppl 1):E20–E30.
Bianco P, Riminucci M, Gronthos S, Robey PG: Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 2001, 19:180–192.
Lecka-Czernik B, Suva LJ: Resolving the two “bony” faces of PPAR-gamma. PPAR Res 2006, 2006:27489.
Evans RM, Barish GD, Wang YX: PPARs and the complex journey to obesity. Nat Med 2004, 10:355–361.
Zoete V, Grosdidier A, Michielin O: Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochim Biophys Acta 2007, 1771:915–925.
Tontonoz P, Spiegelman BM: Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 2008, 77:289–312.
Heikkinen S, Auwerx J, Argmann CA: PPARgamma in human and mouse physiology. Biochim Biophys Acta 2007, 1771:999–1013.
Ren D, Collingwood TN, Rebar EJ, et al.: PPARgamma knockdown by engineered transcription factors: exogenous PPARgamma2 but not PPARgamma1 reactivates adipogenesis. Genes Dev 2002, 16:27–32.
Lecka-Czernik B, Gubrij I, Moerman EA, et al.: Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPAR-gamma 2. J Cell Biochem 1999, 74:357–371.
Willson TM, Lambert MH, Kliewer SA: Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem 2001, 70:341–367.
Knouff C, Auwerx J: Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev 2004, 25:899–918.
Lecka-Czernik B, Moerman EJ, Grant DF, et al.: Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology 2002, 143:2376–2384.
Wan Y, Chong LW, Evans RM: PPAR-gamma regulates osteoclastogenesis in mice. Nat Med 2007, 13:1496–1503.
Rzonca SO, Suva LJ, Gaddy D, et al.: Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 2004, 145:401–406.
• Lazarenko OP, Rzonca SO, Hogue WR, et al.: Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology 2007, 148:2669–2680. This article shows that aging is a confounding factor for rosiglitazone-induced bone loss in mice.
Akune T, Ohba S, Kamekura S, et al.: PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 2004, 113:846–855.
Rosen CJ, Bouxsein ML: Mechanism of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2006, 2:35–43.
Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B: Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 2004, 3:379–389.
Spiteller G: The important role of lipid peroxidation processes in aging and age dependent diseases. Mol Biotechnol 2007, 37:5–12.
Maurin AC, Chavassieux PM, Vericel E, Meunier PJ: Role of polyunsaturated fatty acids in the inhibitory effect of human adipocytes on osteoblastic proliferation. Bone 2002, 31:260–266.
• Shockley KR, Rosen CJ, Churchill GA, Lecka-Czernik B: PPARγ2 regulates a molecular signature of marrow mesenchymal stem cells. PPAR Research 2007, 2007:81219. This article demonstrates that PPAR-γ2 plays a major role in regulation of MSC "stemness" phenotype.
Minaire P, Edouard C, Arlot M, Meunier PJ: Marrow changes in paraplegic patients. Calcif Tissue Int 1984, 36:338–340.
Trudel G, Payne M, Madler B, et al.: Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. J Appl Physiol 2009, 107:540–548.
Suva LJ, Gaddy D, Perrien DS, et al.: Regulation of bone mass by mechanical loading: microarchitecture and genetics. Curr Osteoporos Rep 2005, 3:46–51.
Marie PJ, Kaabeche K: PPAR gamma and control of bone mass in skeletal unloading. PPAR Res 2006, 2006:64807.
Cock TA, Back J, Elefteriou F, et al.: Enhanced bone formation in lipodystrophic PPARgamma(hyp/hyp) mice relocates haematopoiesis to the spleen. EMBO Rep 2004, 5:1007–1012.
Ali AA, Weinstein RS, Stewart SA, et al.: Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology 2005, 146:1226–1235.
Sottile V, Seuwen K, Kneissel M: Enhanced marrow adipogenesis and bone resorption in estrogen-deprived rats treated with the PPARgamma agonist BRL49653 (rosiglitazone). Calcif Tissue Int 2004, 75:329–337.
Modder UI, Monroe DG, Fraser DG, et al.: Skeletal consequences of deletion of steroid receptor coactivator-2/transcription intermediary factor-2. J Biol Chem 2009, 284:18767–18777.
• Shockley KR, Lazarenko OP, Czernik PJ, et al.: PPARγ2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem 2009, 106:232–246. This article provides an extensive analysis of the effect of rosiglitazone on gene expression in marrow MSCs.
Lecka-Czernik B: Bone as a target of type 2 diabetes treatment. Curr Opin Investig Drugs 2009, 10:1085–1090.
• Kahn SE, Zinman B, Lachin JM, et al.: Rosiglitazone-associated fractures in type 2 diabetes: an Analysis from A Diabetes Outcome Progression Trial (ADOPT). Diabetes Care 2008, 31:845–851. This article provides a retrospective analysis of fracture risk and type of fractures among the ADOPT participants.
• Loke YK, Singh S, Furberg CD: Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ 2009, 180:32–39. This article provides a comprehensive review of available clinical trials in respect to the effect of TZDs on risk of fractures.
Meier C, Kraenzlin ME, Bodmer M, et al.: Use of thiazolidinediones and fracture risk. Arch Intern Med 2008, 168:820–825.
Lefebvre P, Chinetti G, Fruchart JC, Staels B: Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest 2006, 116:571–580.
Remick J, Weintraub H, Setton R, et al.: Fibrate therapy: an update. Cardiol Rev 2008, 16:129–141.
Giaginis C, Tsantili-Kakoulidou A, Theocharis S: Peroxisome proliferator-activated receptors (PPARs) in the control of bone metabolism. Fundam Clin Pharmacol 2007, 21:231–244.
Wu X, Peters JM, Gonzalez FJ, et al.: Frequency of stromal lineage colony forming units in bone marrow of peroxisome proliferator-activated receptor-alpha-null mice. Bone 2000, 26:21–26.
Yang Q, Gonzalez FJ: Peroxisome proliferator-activated receptor alpha regulates B lymphocyte development via an indirect pathway in mice. Biochem Pharmacol 2004, 68:2143–2150.
Still K, Grabowski P, Mackie I, et al.: The peroxisome proliferator activator receptor alpha/delta agonists linoleic acid and bezafibrate upregulate osteoblast differentiation and induce periosteal bone formation in vivo. Calcif Tissue Int 2008, 83:285–292.
Chan BY, Gartland A, Wilson PJ, et al.: PPAR agonists modulate human osteoclast formation and activity in vitro. Bone 2007, 40:149–159.
Syversen U, Stunes AK, Gustafsson BI, et al.: Different skeletal effects of the peroxisome proliferator activated receptor (PPAR)alpha agonist fenofibrate and the PPARgamma agonist pioglitazone. BMC Endocr Disord 2009, 9:10.
Meier CR, Schlienger RG, Kraenzlin ME, et al.: HMG-CoA reductase inhibitors and the risk of fractures. JAMA 2000, 283:3205–3210.
Barish GD, Narkar VA, Evans RM: PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest 2006, 116:590–597.
Wang YX, Lee CH, Tiep S, et al.: Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell 2003, 113:159–170.
Gimble JM, Zvonic S, Floyd ZE, et al.: Playing with bone and fat. J Cell Biochem 2006, 98:251–266.
Tavassoli M: Marrow adipose cells and hemopoiesis: an interpretative review. Exp Hematol 1984, 12:139–146.
Elefteriou F, Ahn JD, Takeda S, et al.: Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 2005, 434:514–520.
Shinoda Y, Yamaguchi M, Ogata N, et al.: Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem 2006, 99:196–208.
Cartwright MJ, Tchkonia T, Kirkland JL: Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Exp Gerontol 2007, 42:463–471.
Gasparrini M, Rivas D, Elbaz A, Duque G: Differential expression of cytokines in subcutaneous and marrow fat of aging C57BL/6 J mice. Exp Gerontol 2009, 44:613–618.
Disclosure
Dr. Beata Lecka-Czernik has received support from funds from the US National Institutes of Health/National Institute on Aging (NIA AG 028935) and American Diabetes Association’s Amaranth Diabetes Fund 1-09-RA-95.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lecka-Czernik, B. PPARs in Bone: The Role in Bone Cell Differentiation and Regulation of Energy Metabolism. Curr Osteoporos Rep 8, 84–90 (2010). https://doi.org/10.1007/s11914-010-0016-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-010-0016-1