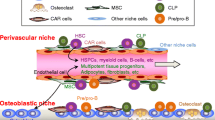
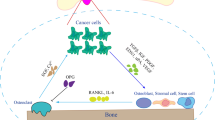
Abstract
The metastasis of tumor cells to distant organs is the primary cause of cancer-related mortality in most cancers. The interaction of tumor cells with local stroma at the metastatic site plays a critical role in metastatic dissemination and the establishment of metastases. These tumor-stromal interactions regulate several important steps including degradation of extracellular matrix, release of sequestered growth factors, and expression of chemokines, cytokines, and receptors on tumor cells and the interacting stromal cells. Breast, prostate, and lung cancers preferentially metastasize to bone. Tumor cell interactions with the bone microenvironment initiate a series of complex cellular interactions that promotes establishment of osteoclastic and/or osteoblastic metastasis. Understanding the interactions between tumor cells and the stroma is important to identify molecular targets to develop novel therapies aimed at reducing metastasis formation. In this article, we review the important mechanisms of tumor-stromal interaction in the development of bone metastasis.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance
Paget S: The distribution of secondary growths in cancer of the breast. Lancet 1889, 133:571–573.
Fidler IJ, Kripke ML: Metastasis results from preexisting variant cells within a malignant tumor. Science 1977, 197:893–895.
Hart IR, Fidler IJ: Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res 1980, 40:2281–2287.
• Fidler IJ, Poste G: The "seed and soil" hypothesis revisited. Lancet Oncol 2008, 9:808. This is an important review of the well-known "seed and soil" hypothesis in metastasis with reference to the latest understanding of metastatic determinants.
•• Suva LJ, Griffin RJ, Makhoul I: Mechanisms of bone metastases of breast cancer. Endocr Relat Cancer 2009, 16:703–713. This paper highlights the underlying mechanisms of breast cancer bone metastasis.
Leong SP, Cady B, Jablons DM, et al.: Clinical patterns of metastasis. Cancer Metastasis Rev 2006, 25:221–232.
•• Joyce JA, Pollard JW: Microenvironmental regulation of metastasis. Nat Rev Cancer 2009, 9:239–252. This comprehensive review highlights the role of the microenvironment in metastasis regulation.
Bussard KM, Gay CV, Mastro AM: The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev 2008, 27:41–55.
Mundy GR: Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002, 2:584–593.
Lester J, Dodwell D, McCloskey E, Coleman R: The causes and treatment of bone loss associated with carcinoma of the breast. Cancer Treat Rev 2005, 31:115–142.
Gabbitas B, Canalis E: Growth factor regulation of insulin-like growth factor binding protein-6 expression in osteoblasts. J Cell Biochem 1997, 66:77–86.
Kakonen SM, Mundy GR: Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer 2003, 97:834–839.
Birkedal-Hansen H: Catabolism and turnover of collagens: collagenases. Methods Enzymol 1987, 144:140–171.
Leppa S, Saarto T, Vehmanen L, et al.: A high serum matrix metalloproteinase-2 level is associated with an adverse prognosis in node-positive breast carcinoma. Clin Cancer Res 2004, 10:1057–1063.
Saad S, Bendall LJ, James A, et al.: Induction of matrix metalloproteinases MMP-1 and MMP-2 by co-culture of breast cancer cells and bone marrow fibroblasts. Breast Cancer Res Treat 2000, 63:105–115.
Zhao W, Byrne MH, Boyce BF, Krane SM: Bone resorption induced by parathyroid hormone is strikingly diminished in collagenase-resistant mutant mice. J Clin Invest 1999, 103:517–524.
• Wilson TJ, Singh RK: Proteases as modulators of tumor-stromal interaction: primary tumors to bone metastases. Biochim Biophys Acta 2008, 1785:85–95. This is an interesting review of proteases and their role in tumor-stromal interaction.
Coleman RE: Risks and benefits of bisphosphonates. Br J Cancer 2008, 98:1736–1740.
Ohshiba T, Miyaura C, Inada M, Ito A: Role of RANKL-induced osteoclast formation and MMP-dependent matrix degradation in bone destruction by breast cancer metastasis. Br J Cancer 2003, 88:1318–1326.
Yoneda T, Sasaki A, Dunstan C, et al.: Inhibition of osteolytic bone metastasis of breast cancer by combined treatment with the bisphosphonate ibandronate and tissue inhibitor of the matrix metalloproteinase-2. J Clin Invest 1997, 99:2509–2517.
Sheng S, Carey J, Seftor EA, et al.: Maspin acts at the cell membrane to inhibit invasion and motility of mammary and prostatic cancer cells. Proc Natl Acad Sci U S A 1996, 93:11669–11674.
Cher ML, Biliran Jr HR, Bhagat S, et al.: Maspin expression inhibits osteolysis, tumor growth, and angiogenesis in a model of prostate cancer bone metastasis. Proc Natl Acad Sci U S A 2003, 100:7847–7852.
Lynch CC, Hikosaka A, Acuff HB, et al.: MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell 2005, 7:485–496.
Dong Z, Bonfil RD, Chinni S, et al.: Matrix metalloproteinase activity and osteoclasts in experimental prostate cancer bone metastasis tissue. Am J Pathol 2005, 166:1173–1186.
Ishibashi O, Niwa S, Kadoyama K, Inui T: MMP-9 antisense oligodeoxynucleotide exerts an inhibitory effect on osteoclastic bone resorption by suppressing cell migration. Life Sci 206, 79:1657–1660.
Ishikawa T, Kamiyama M, Tani-Ishii N, et al.: Inhibition of osteoclast differentiation and bone resorption by cathepsin K antisense oligonucleotides. Mol Carcinog 2001, 32:84–91.
Wilson TJ, Nannuru KC, Futakuchi M, et al.: Cathepsin G enhances mammary tumor-induced osteolysis by generating soluble receptor activator of nuclear factor-{kappa}B Ligand. Cancer Res 2008, 68:5803–5811.
Wilson TJ, Nannuru KC, Singh RK: Cathepsin G recruits osteoclast precursors via proteolytic activation of protease-activated receptor-1. Cancer Res 2009, 69:3188–3195.
•• Wilson TJ, Nannuru KC, Futakuchi M, Singh RK: Cathepsin G-mediated enhanced TGF-beta signaling promotes angiogenesis via upregulation of VEGF and MCP-1. Cancer Lett 2010, 288:162–169. This is an important study defining the novel role of cathepsin G in TGF-β signaling and angiogenesis.
Thomas RJ, Guise TA, Yin JJ, et al.: Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology 1999, 140:4451–4458.
Yin JJ, Selander K, Chirgwin JM, et al.: TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest 1999, 103:197–206.
Bendre MS, Margulies AG, Walser B, et al.: Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res 2005, 65:11001–11009.
• Thiolloy S, Halpern J, Holt GE, et al.: Osteoclast-derived matrix metalloproteinase-7, but not matrix metalloproteinase-9, contributes to tumor-induced osteolysis. Cancer Res 2009, 69:6747–6755. This is an important study delineating the role of proteases in osteolytic tumor using knockout mouse models.
Mengshol JA, Vincenti MP, Brinckerhoff CE: IL-1 induces collagenase-3 (MMP-13) promoter activity in stably transfected chondrocytic cells: requirement for Runx-2 and activation by p38 MAPK and JNK pathways. Nucleic Acids Res 2001, 29:4361–4372.
Sakamoto H, Ochiya T, Sato Y, et al.: Adenovirus-mediated transfer of the HST-1 (FGF4) gene induces increased levels of platelet count in vivo. Proc Natl Acad Sci U S A 1994, 91:12368–12372.
Guise TA, Kozlow WM, Heras-Herzig A, et al.: Molecular mechanisms of breast cancer metastases to bone. Clin Breast Cancer 2005, 5(Suppl):S46–S53.
Koeneman KS, Yeung F, Chung LW: Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate 1999, 39:246–261.
• Wilson TJ, Nannuru KC, Singh RK: Cathepsin G-mediated activation of pro-matrix metalloproteinase 9 at the tumor-bone interface promotes transforming growth factor-beta signaling and bone destruction. Mol Cancer Res 2009, 7:1224–1233. This important study demonstrates the interplay of cathepsin G, TGF-β, and MMP-9 in tumor-induced osteolysis.
Massagué J: TGFbeta in cancer. Cell 2008, 34:215–230.
Harris SE, Bonewald LF, Harris MA, et al.: Effects of transforming growth factor beta on bone nodule formation and expression of bone morphogenetic protein 2, osteocalcin, osteopontin, alkaline phosphatase, and type I collagen mRNA in long-term cultures of fetal rat calvarial osteoblasts. J Bone Miner Res 1994, 9:855–863.
Futakuchi M, Nannuru KC, Varney ML, et al.: Transforming growth factor-beta signaling at the tumor-bone interface promotes mammary tumor growth and osteoclast activation. Cancer Sci 2009, 100:71–81.
Dunn LK, Mohammad KS, Fournier PG, et al.: Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One 2009, 4:e6896.
Nemeth JA, Yousif R, Herzog M, et al.: Matrix metalloproteinase activity, bone matrix turnover, and tumor cell proliferation in prostate cancer bone metastasis. J. Natl Cancer Inst 2002, 94:17–25.
Mitsiades CS, Mitsiades NS, McMullan CJ, et al.: Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell 2004, 5:221–230.
Doerr ME, Jones JI: The roles of integrins and extracellular matrix proteins in the insulin-like growth factor I-stimulated chemotaxis of human breast cancer cells. J Biol Chem 1996, 271:2443–2447.
Takuwa Y, Masaki T, Yamashita K: The effects of the endothelin family peptides on cultured osteoblastic cells from rat calvariae. Biochem Biophys Res Commun 1990, 170:998–1005.
Guise TA, Chirgwin JM: Transforming growth factor-beta in osteolytic breast cancer bone metastases. Clin Orthop Relat Res 2003, 415(Suppl):S32–S38.
Chiao JW, Moonga BS, Yang YM, et al.: Endothelin-1 from prostate cancer cells is enhanced by bone contact which blocks osteoclastic bone resorption. Br J Cancer 2000, 83:360–365.
• Singh S, Sadanandam A, Singh RK: Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis Rev 2007, 26:453–467. This paper highlights the role of chemokines in tumor angiogenesis and metastasis.
Murphy PM: Chemokines and the molecular basis of cancer metastasis. N Engl J Med 2001, 345:833–835.
Muller A, Homey B, Soto H, et al.: Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410:50–56.
Nakamura ES, Koizumi K, Kobayashi M, et al.: RANKL-induced CCL22/macrophage-derived chemokine produced from osteoclasts potentially promotes the bone metastasis of lung cancer expressing its receptor CCR4. Clin Exp Metastasis 2006, 23:9–18.
Chinni SR, Sivalogan S, Dong Z, et al.: CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate 2006, 66:32–48.
Takahashi M, Miyazaki H, Furihata M, et al.: Chemokine CCL2/MCP-1 negatively regulates metastasis in a highly bone marrow-metastatic mouse breast cancer model. Clin Exp Metastasis 2009, 26:817–828.
Pantel K, Alix-Panabieres C, Riethdorf S: Cancer micrometastases. Nat Rev Clin Oncol 2009, 6:339–351.
Acknowledgments
This work was supported in part by Susan G. Komen for the Cure grant KG090860, Cancer Glycobiology Program from Nebraska Research Initiative and by grant CA72781 (Dr. Rakesh K. Singh), and a Cancer Center Support Grant (P30CA036727) from the National Cancer Institute, National Institutes of Health, and the Department of Defense (DOD) (Breast Cancer Research Program [BCRP] Predoctoral Traineeship Award [BC083293]) (Dr. Kalyan C. Nannuru).
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nannuru, K.C., Singh, R.K. Tumor-Stromal Interactions in Bone Metastasis. Curr Osteoporos Rep 8, 105–113 (2010). https://doi.org/10.1007/s11914-010-0011-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-010-0011-6