Abstract
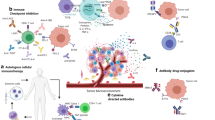
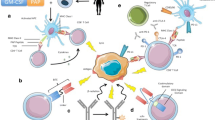
Whereas androgen deprivation and chemotherapy have become the cornerstone of therapy for advanced prostate cancer, novel therapies are being developed that may expand upon currently available treatments. The identification of antigens expressed by prostate tissue and/or prostate cancer that are recognized by T cells or antibodies creates opportunities to develop novel immunotherapeutic approaches including tumor vaccines. Proteins expressed in prostate cancer—including prostate-specific antigen, prostatic acid phosphatase, and prostate membrane antigen—have been used as immunologic targets for immunotherapy. Moreover, innovations in cancer genomics and proteomics also will aid in the identification of immunologic targets. Emerging trials have demonstrated that immunotherapy may generate not only immune responses in patients but also clinical responses. Future studies will be directed at capitalizing on these findings.
Similar content being viewed by others
References and Recommended Reading
Wang X, Yu J, Sreekumar A, et al.: Autoantibody signatures in prostate cancer. N Engl J Med 2005, 53:1224–1235.
Van Parijs L, Abbas AK: Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science 1998, 280:243–248.
Fong L, Engleman EG: Dendritic Cells in cancer immunotherapy. Annu Rev Immunol 2000, 18:245–273.
Hudson MA, Bahnson RR, Catalona WJ: Clinical use of prostate-specific antigen in patients with prostate cancer. J Urol 1989, 142:1011–1017.
Plowman PN, Perry LA, Chard T: Androgen suppression by hydrocortisone without aminoglutethimide in orchiectomised men with prostatic cancer. Br J Urol 1987, 59:255–257.
Storlie JA, Buckner JC, Wiseman GA, et al.: Prostate-specific antigen levels and clinical response to low-dose dexamethasone for hormone-refractory metastatic prostate carcinoma. Cancer 1995, 76:96–100.
Small EJ, McMillan A, Meyer M, et al.: Serum prostate-specific antigen decline as a marker of clinical outcome in hormone-refractory prostate cancer patients: association with progression-free survival, pain end points, and survival. J Clin Oncol 2001, 19:1304–1311.
Schmid HP, McNeal JE, Stamey TA: Observations on the doubling time of prostate cancer the use of serial prostate-specific antigen in patients with untreated disease as a measure of increasing cancer volume. Cancer 1993, 71:2031–2040.
Slovin SF, Scher HI: Peptide and carbohydrate vaccines in relapsed prostate cancer: immunogenicity of synthetic vaccines in man—clinical trials at Memorial Sloan-Kettering Cancer Center. Semin Oncol 1999, 26:448–454.
Fong L, Brockstedt D, Benike C, et al.: Dendritic cell-based xenoantigen vaccination for prostate cancer immunotherapy. J Immunol 2001, 167:7150–7156.
Rini BI, Weinberg V, Bok R, et al.: Prostate-specific antigen kinetics as a measure of the biologic effect of granulocyte-macrophage colony-stimulating factor in patients with serologic progression of prostate cancer. J Clin Oncol 2003, 21:99–105.
Heiser A, Coleman D, Dannull J, et al.: Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest 2002, 109:409–417.
Yamamoto JK, Kruzel ML, Louie H, et al.: Inhibition of human immunodeficiency virus type-1 replication by human interferons alpha, beta, and gamma. Arch Immunol Ther Exp 1993, 41:185–191.
Savage PA, Boniface JJ, Davis MM: A kinetic basis for T-cell receptor repertoire selection during an immune response. Immunity 1999, 10:485–492.
Correale P, Walmsley K, Nieroda C, et al.: In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J Natl Cancer Inst 1997, 89:293–300.
Xue BH, Zhang Y, Sosman JA, et al.: Induction of human cytotoxic T lymphocytes specific for prostate-specific antigen. Prostate 1997, 30:73–78.
Sanda MG, Smith DC, Charles LG, et al.: Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology 1999, 53:260–266.
Eder JP, Kantoff PW, Roper K, et al.: A phase-I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res 2000, 6:1632–1638.
Gulley J, Chen AP, Dahut W, et al.: Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate 2002, 53:109–117.
Vihko P, Virkkunen P, Henttu P, et al.: Molecular cloning and sequence analysis of cDNA encoding human prostatic acid phosphatase. FEBS Lett 1988, 236:275–281.
Fong L, Benike C, Brockstedt D, et al.: Immunization with dendritic cells pulsed with xenogeneic prostatic acid phosphatase administered via different routes induces cellular immune responses in prostate cancer patients. Proc Amer Assoc Cancer Res 1999, 40:85.
Small EJ, Fratesi P, Reese DM, et al.: Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol 2000, 18:3894–3903.
Small EJ, Rini B, Higano C, et al.: A randomized, placebo-controlled phase iii trial of APC8015 (Provenge™) in patients with androgen-independent prostate cancer (AiPCa) [Abstract 1534]. Presented at the 39th Annual Meeting of the American Society of Clinical Oncology. Chicago: May 31–June 3, 2003.
Small EJ, Schellhammer PF, Higano C, et al.: Immunotherapy (APC8015) for androgen-independent prostate cancer (AIPC): final survival data from a phase 3 randomized placebo-controlled trial. Paper presented at the 2005 Prostate Cancer Symposium. Orlando: February 17–19, 2005.
Lu J, Celis E: Recognition of prostate tumor cells by cytotoxic T lymphocytes specific for prostate-specific membrane antigen. Cancer Res 2002, 62:5807–5812.
Murphy G, Tjoa B, Ragde H, et al.: Phase-I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate 1996, 29:371–380.
Murphy GP, Tjoa BA, Simmons SJ, et al.: Phase-II prostate cancer vaccine trial: report of a study involving 37 patients with disease recurrence following primary treatment. Prostate 1999, 39:54–59.
Tjoa BA, Simmons SJ, Elgamal A, et al.: Follow-up evaluation of a phase-II prostate cancer vaccine trial. Prostate 1999, 40:125–129.
Simons JW, Mikhak B, Chang JF, et al.: Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res 1999, 59:5160–5168.
Mincheff M, Tchakarov S, Zoubak S, et al.: Naked DNA and adenoviral immunizations for immunotherapy of prostate cancer: a phase I/II clinical trial. Eur Urol 2000, 38:208–217.
Nair SK, Heiser A, Boczkowski D, et al.: Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nat Med 2000, 6:1011–1017.
Su Z, Dannull J, Yang BK, et al.: Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol 2005, 174:3798–3807.
Vonderheide RH, Domchek SM, Schultze JL, et al.: Vaccination of cancer patients against telomerase induces functional anti-tumor CD8+ T lymphocytes. Clin Cancer Res 2004, 10:828–839.
Agus DB, Scher HI, Higgins B, et al.: Response of prostate cancer to anti-Her-2/neu antibody in androgen-dependent and-independent human xenograft models. Cancer Res 1999, 59:4761–4764.
Reese DM, Small EJ, Magrane G, et al.: HER2 protein expression and gene amplification in androgen-independent prostate cancer. Am J Clin Pathol 2001, 116:234–239.
McDevitt MR, Barendswaard E, Ma D, et al.: An alpha-particle emitting antibody ([213Bi]J591) for radioimmunotherapy of prostate cancer. Cancer Res 2000, 60:6095–6100.
Fracasso G, Bellisola G, Cingarlini S, et al.: Anti-tumor effects of toxins targeted to the prostate specific membrane antigen. Prostate 2002, 53:9–23.
Deb N, Goris M, Trisler K, et al.: Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin Cancer Res 1996, 2:1289–1297.
Bander NH, Trabulsi EJ, Yao D, et al.: Phase-I radioimmunotherapy (RIT) trials of humanized monoclonal antibody (mAb) J591 to the extracellular domain of prostate specific membrane antigen (PSMA ext) radiolabeled with 90 yttrium (90Y) or 177 lutetium (177Lu) in advanced prostate cancer (Pca). Proc Am Soc Clin Oncol 2002, 21:5a.
Alvarez RD, Partridge EE, Khazaeli MB, et al.: Intraperitoneal radioimmunotherapy of ovarian cancer with 177Lu-CC49: a phase-I/II study. Gynecol Oncol 1997, 65:94–101.
Myers RB, Meredith RF, Schlom J, et al.: Tumor-associated glycoprotein-72 is highly expressed in prostatic adenocarcinomas. J Urol 1994, 152:243–246.
Slovin SF, Scher HI, Divgi CR, et al.: Interferon-gamma and monoclonal antibody 131I-labeled CC49: outcomes in patients with androgen-independent prostate cancer. Clin Cancer Res 1998, 4:643–651.
Meredith RF, Bueschen AJ, Khazaeli MB, et al.: Treatment of metastatic prostate carcinoma with radiolabeled antibody CC49. J Nucl Med 1994, 35:1017–1022.
Meredith RF, Khazaeli MB, Macey DJ, et al.: Phase-II study of interferon-enhanced 131I-labeled high affinity CC49 monoclonal antibody therapy in patients with metastatic prostate cancer. Clin Cancer Res 1999, 5:3254s–3258s.
Katzenwadel A, Schleer H, Gierschner D, et al.: Construction and in vivo evaluation of an anti-PSA × anti-CD3 bispecific antibody for the immunotherapy of prostate cancer. Anticancer Res 2000, 20:1551–1555.
Slovin SF, Ragupathi G, Adluri S, et al.: Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc Natl Acad Sci U S A 1999, 96:5710–5715.
Dranoff G, Jaffee E, Lazenby A, et al.: Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A 1993, 90:3539–3543.
Baskar S, Glimcher L, Nabavi N, et al.: Major histocompatibility complex class II+B7-1+ tumor cells are potent vaccines for stimulating tumor rejection in tumor-bearing mice. J Exp Med 1995, 181:619–629.
Simons J, Nelson W, Nemunaitis J, et al.: Phase-II trials of a GM-CSF gene-transduced prostate cancer cell line vaccine (GVAX) in hormone refractory prostate cancer [Abstract 729]. Proc Am Soc Clin Oncol 2002, 21:183a.
Sacks N, Small E, Higano C, et al.: A phase I/II study of high dose allogeneic GM-CSF gene-transduced prostate cancer cell line vaccine in patients with metastatic hormone-refractory prostate cancer. Mol Ther 2003, 7:S447.
Small EJ, Reese DM, Um B, et al.: Therapy of advanced prostate cancer with granulocyte macrophage colony-stimulating factor. Clin Cancer Res 1999, 5:1738–1744.
Egen JG, Kuhns MS, Allison JP: CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 2002, 3:611–618.
Hurwitz AA, Foster BA, Kwon ED, et al.: Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res 2000, 60:2444–2448.
Davis TA, Tchekmedyian S, Korman A, et al.: MDX-010 (human anti-CTLA4): a phase 1 trial in hormone refractory prostate carcinoma (HRPC) [Abstract 74]. Proc Am Soc Clin Oncol 2002.
Mercader M, Bodner BK, Moser MT, et al.: T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A 2001, 98:14565–14570.
Sutherland JS, Goldberg GL, Hammett MV, et al.: Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol 2005, 175:2741–2753.
Drake CG, Doody AD, Mihalyo MA, et al.: Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell 2005, 7:239–249.
Dannull J, Diener PA, Prikler L, et al.: Prostate stem cell antigen is a promising candidate for immunotherapy of advanced prostate cancer. Cancer Res 2000, 60:5522–5528.
Kiessling A, Schmitz M, Stevanovic S, et al.: Prostate stem cell antigen: identification of immunogenic peptides and assessment of reactive CD8+ T cells in prostate cancer patients. Int J Cancer 2002, 102:390–397.
Tsavaler L, Shapero MH, Morkowski S, et al.: Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res 2001, 61:3760–3769.
Kiessling A, Stevanovic S, Fussel S, et al.: Identification of an HLA-A*0201-restricted T-cell epitope derived from the prostate cancer-associated protein prostein. Br J Cancer 2004, 90:1034–1040.
Alves PM, Faure O, Graff-Dubois S, et al.: EphA2 as target of anticancer immunotherapy: identification of HLA-A*0201-restricted epitopes. Cancer Res 2003, 63:8476–8480
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fong, L., Small, E.J. Immunotherapy for prostate cancer. Curr Oncol Rep 9, 226–233 (2007). https://doi.org/10.1007/s11912-007-0026-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-007-0026-z