Abstract
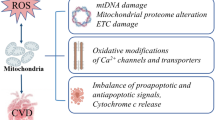
Mitochondria are the primary generators of cellular reactive oxygen species (ROS); their pathophysiological roles in hypertension and insulin resistance are but imperfectly understood. Mitochondrial dysfunction has been linked to the etiologies of many complex diseases, but many other factors, including the upregulation of the renin-angiotensin system (RAS) and vitamin D deficiency, have also been implicated in hypertension pathogenesis. Hypertension resulting from the disruption of the RAS contributes to the risk of cardiovascular disease. Likewise, experimental and clinical evidence indicate that RAS stimulation and low vitamin D levels are inversely related and represent risk factors associated with the pathogenesis of hypertension. Furthermore, RAS activation induces insulin resistance, resulting in increases in ROS levels. High levels of ROS are harmful to cells, having the potential to trigger both mitochondrial-mediated apoptosis and the degradation of the mitochondrial DNA. Diabetes risk is also associated with high levels of oxidative stress; taking vitamin D, however, may reduce that risk. The finding that mitochondria possess both a functional RAS and vitamin D receptors is the starting point for improving our understanding of the interaction of mitochondria and chronic disease states, which understanding should lead to decreases in the chronic disease burden attributable to hypertension, diabetes, or both.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Henze K, Martin W. Evolutionary biology: essence of mitochondria. Nature. 2003;426(6963):127–8.
McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16(14):R551–60.
Gardner A, Boles RG. Is a “mitochondrial psychiatry” in the future? A review. Curr Psychiatr Rev. 2005;1(3):255–71.
Lesnefsky EJ, Moghaddas S, Tandler B, Kerner B, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia-reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33(6):1065–89.
Dikalov SI, Ungvari Z. Role of mitochondrial oxidative stress in hypertension. Am J Physiol Heart Circ Physiol. 2013;305(10):H1417–27.
Min B. Effects of vitamin D on blood pressure and endothelial function. Korean J Physiol Pharmacol. 2013;17(5):385–92.
Ferder M, Inserra F, Manucha W, Ferder L. The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin-angiotensin system. Am J Physiol Cell Physiol. 2013;304(11):C1027–39.
Montezano AC, Touyz RM. Molecular mechanisms of hypertension-reactive oxygen species and antioxidants: a basic science update for the clinician. Can J Cardiol. 2012;28(3):288–95.
Wen H, Gwathmey JK, Xie LH. Oxidative stress-mediated effects of angiotensin II in the cardiovascular system. World J Hypertens. 2012;2(4):34–44.
Gunta SS, Thadhani RI, Mak RH. The effect of vitamin D status on risk factors for cardiovascular disease. Nat Rev Nephrol. 2013;9(6):337–47.
Skov J, Persson F, Frøkiær J, Christiansen JS. Tissue renin-angiotensin systems: a unifying hypothesis of metabolic disease. Front Endocrinol (Lausanne). 2014;5:23.
Vaidya A, Williams JS. The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism. 2012;61(4):450–8.
Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89–90(1–5):387–92.
Silvagno F, De Vivo E, Attanasio A, Gallo V, Mazzucco G, Pescarmona G. Mitochondrial localization of vitamin D receptor in human platelets and differentiated megakaryocytes. PLoS ONE. 2010;5(1):e8670.
García IM, Altamirano L, Mazzei L, Fornés M, Molina MN, Ferder L, et al. Role of mitochondria in paricalcitol-mediated cytoprotection during obstructive nephropathy. Am J Physiol Renal Physiol. 2012;302(12):F1595–605.
Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, et al. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A. 2011;108(36):14849–54.
Dong J, Wong SL, Lau CW, Lee HK, Ng CF, Zhang L, et al. Calcitriol protects renovascular function in hypertension by down-regulating angiotensin II type 1 receptors and reducing oxidative stress. Eur Heart J. 2012;33(23):2980–90.
García IM, Altamirano L, Mazzei L, Fornés M, Cuello-Carrión FD, Ferder L, et al. Vitamin D receptor-modulated Hsp70/AT1 expression may protect the kidneys of SHRs at the structural and functional levels. Cell Stress Chaperones. 2014;19(4):479–91.
Manrique C, Lastra G, Gardner M, Sowers JR. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin N Am. 2009;93(3):569–82.
Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51(5):993–9.
Vázquez-Medina JP, Popovich I, Thorwald MA, Viscarra JA, Rodriguez R, Sonanez-Organis JG, et al. Angiotensin receptor-mediated oxidative stress is associated with impaired cardiac redox signaling and mitochondrial function in insulin-resistant rats. Am J Physiol Heart Circ Physiol. 2013;305(4):H599–607.
Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med (Berl). 2010;88(10):993–1001.
Mitri J, Pittas AG. Vitamin D and diabetes. Endocrinol Metab Clin N Am. 2014;43(1):205–32.
Mirzaei K, Hossein-Nezhad A, Keshavarz SA, Eshaghi SM, Koohdani F, Saboor-Yaraghi AA, et al. Insulin resistance via modification of PGC1α function identifying a possible preventive role of vitamin D analogues in chronic inflammatory state of obesity. A double blind clinical trial study. Minerva Med. 2014;105(1):63–78.
Dutta D, Maisnam I, Shrivastava A, Sinha A, Ghosh S, Mukhopadhyay P, et al. Serum vitamin-D predicts insulin resistance in individuals with prediabetes. Indian J Med Res. 2013;138(6):853–60.
Bonakdaran S, Nejad AF, Abdol-Reza V, Hatefi A, Shakeri M. Impact of oral 1,25-dihydroxy vitamin d (calcitriol) replacement therapy on coronary artery risk factors in type 2 diabetic patients. Endocr Metab Immune Disord Drug Targets. 2013;13(4):295–300.
Ullah MI, Uwaifo GI, Nicholas WC, Koch CA. Does vitamin d deficiency cause hypertension? Current evidence from clinical studies and potential mechanisms. Int J Endocrinol. 2010. doi:10.1155/2010/579640.
Hess R, Pearse AG. Mitochondrial alpha-glycerophosphate dehydrogenase activity of juxtaglomerular cells in experimental hypertension and adrenal insufficiency. Proc Soc Exp Biol Med. 1961;106:895–8.
Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93(8):903–7.
Hernanz R, Briones AM, Salaices M, Alonso MJ. New roles for old pathways? A circuitous relationship between reactive oxygen species and cyclo-oxygenase in hypertension. Clin Sci (Lond). 2014;126(2):111–21.
Maulik SK, Kumar S. Oxidative stress and cardiac hypertrophy: a review. Toxicol Mech Methods. 2012;22(5):359–66.
Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51(7):1289–301.
de Cavanagh EM, Ferder M, Inserra F, Ferder L. Angiotensin II, mitochondria, cytoskeletal, and extracellular matrix connections: an integrating viewpoint. Am J Physiol Heart Circ Physiol. 2009;296:H550–8.
Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102(4):488–96.
de Cavanagh EM, Inserra F, Ferder L. Angiotensin II blockade: a strategy to slow ageing by protecting mitochondria? Cardiovasc Res. 2011;89(1):31–40.
de Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol. 2007;27(6):545–53.
Piotrkowski B, Koch OR, De Cavanagh EM, Fraga CG. Cardiac mitochondrial function and tissue remodelling are improved by a non-antihypertensive dose of enalapril in spontaneously hypertensive rats. Free Radic Res. 2009;43(4):390–9.
Kawashima H. Altered vitamin D metabolism in the kidney of the spontaneously hypertensive rat. Biochem J. 1986;237(3):893–7.
Chun R, Gacad MA, Hewison M, Adams JS. Adenosine 5′-triphosphate-dependent vitamin D sterol binding to heat shock protein-70 chaperones. Endocrinology. 2005;146(12):5540–4.
Pons H, Ferrebuz A, Quiroz Y, Romero-Vasquez F, Parra G, Johnson RJ, et al. Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2013;304(3):F289–99.
Chanoux RA, Robay A, Shubin CB, Kebler C, Suaud L, Rubenstein RC. Hsp70 promotes epithelial sodium channel functional expression by increasing its association with coat complex II and its exit from endoplasmic reticulum. J Biol Chem. 2012;287(23):19255–65.
Needham PG, Mikoluk K, Dhakarwal P, Khadem S, Snyder AC, Subramanya AR, et al. The thiazide-sensitive NaCl cotransporter is targeted for chaperone-dependent endoplasmic reticulum-associated degradation. J Biol Chem. 2011;286(51):43611–21.
Bocanegra V, Manucha W, Peña MR, Cacciamani V, Vallés PG. Caveolin-1 and Hsp70 interaction in microdissected proximal tubules from spontaneously hypertensive rats as an effect of Losartan. J Hypertens. 2010;28(1):143–55.
Ma J, Farmer KL, Pan P, Urban MJ, Zhao H, Blagg BS, et al. Heat shock protein 70 is necessary to improve mitochondrial bioenergetics and reverse diabetic sensory neuropathy following KU-32 therapy. J Pharmacol Exp Ther. 2014;348(2):281–92.
Deocaris CC, Kaul SC, Wadhwa R. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones. 2006;11(2):116–28.
Bottoni P, Giardina B, Pontoglio A, Scarà S, Scatena R. Mitochondrial proteomic approaches for new potential diagnostic and prognostic biomarkers in cancer. Adv Exp Med Biol. 2012;942:423–40.
Jelenik T, Roden M. Mitochondrial plasticity in obesity and diabetes mellitus. Antioxid Redox Signal. 2013;19(3):258–68.
Lee HK. Evidence that the mitochondrial genome is the thrifty genome. Diabetes Res Clin Pract. 1999;45(2–3):127–35.
Padmalayam I. Targeting mitochondrial oxidative stress through lipoic acid synthase: a novel strategy to manage diabetic cardiovascular disease. Cardiovasc Hematol Agents Med Chem. 2012;10(3):223–33.
Puddu P, Puddu GM, Cravero E, De Pascalis S, Muscari A. The emerging role of cardiovascular risk factor-induced mitochondrial dysfunction in atherogenesis. J Biomed Sci. 2009;16:112.
Stump CS, Henriksen EJ, Wei Y, Sowers JR. The metabolic syndrome: role of skeletal muscle metabolism. Ann Med. 2006;38(6):389–402.
Mori J, Zhang L, Oudit GY, Lopaschuk GD. Impact of the renin-angiotensin system on cardiac energy metabolism in heart failure. J Mol Cell Cardiol. 2013;63:98–106.
Finckenberg P, Eriksson O, Baumann M, Merasto S, Lalowski MM, Levijoki J, et al. Caloric restriction ameliorates angiotensin II-induced mitochondrial remodeling and cardiac hypertrophy. Hypertension. 2012;59(1):76–84.
Koroshi A, Idrizi A. Renoprotective effects of Vitamin D and renin-angiotensin system. Hippokratia. 2011;15(4):308–11.
Cheng Q, Boucher BJ, Leung PS. Modulation of hypovitaminosis D-induced islet dysfunction and insulin resistance through direct suppression of the pancreatic islet renin-angiotensin system in mice. Diabetologia. 2013;56(3):553–62.
Guzey M, Takayama S, Reed JC. BAG1L enhances trans-activation function of the vitamin D receptor. J Biol Chem. 2000;275(52):40749–56.
Korányi L, Hegedüs E, Péterfal E, Kurucz I. The role of hsp60 and hsp70 kDa heat shock protein families in different types of diabetes mellitus. Orv Hetil. 2004;145(9):467–72.
Chichester L, Wylie AT, Craft S, Kavanagh K. Muscle heat shock protein 70 predicts insulin resistance with aging. J Gerontol A Biol Sci Med Sci. 2014.
Baseler WA, Dabkowski ER, Williamson CL, Croston TL, Thapa D, Powell MJ, et al. Proteomic alterations of distinct mitochondrial subpopulations in the type 1 diabetic heart: contribution of protein import dysfunction. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R186–200.
Kondo T, Koga S, Matsuyama R, Miyagawa K, Goto R, Kai H, et al. Heat shock response regulates insulin sensitivity and glucose homeostasis: pathophysiological impact and therapeutic potential. Curr Diabetes Rev. 2011;7(4):264–9.
Hooper PL, Balogh G, Rivas E, Kavanagh K, Vigh L. The importance of the cellular stress response in the pathogenesis and treatment of type 2 diabetes. Cell Stress Chaperones. 2014.
Compliance with Ethics Guidelines
Conflict of Interest
Walter Manucha, Bob Richie, and León Ferder declare no conflicts of interest.
Human and Animal Rights and Informed Consent
The animal experiments described herein were not performed by any of the authors, signifying that no animals were harmed in the course of preparing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Hypertension and Obesity
Rights and permissions
About this article
Cite this article
Manucha, W., Ritchie, B. & Ferder, L. Hypertension and Insulin Resistance: Implications of Mitochondrial Dysfunction. Curr Hypertens Rep 17, 504 (2015). https://doi.org/10.1007/s11906-014-0504-2
Published:
DOI: https://doi.org/10.1007/s11906-014-0504-2