Abstract
Chronic hepatitis B infection confers a major risk of cirrhosis and hepatocellular carcinoma worldwide. The ultimate long-term goal of chronic hepatitis B therapy is to reduce morbidity and mortality related to liver disease progression. The ideal treatment endpoint is HBsAg seroconversion. As HBsAg clearance is rarely achieved, the short-term goal of therapy is maintained suppression of hepatitis B replication. For HBeAg-positive patients, durable HBeAg seroconversion with undetectable HBVDNA is a satisfactory intermediate end-point. For HBeAg-negative patients, the treatment endpoint remains unclear, hence, sustained suppression of HBV DNA level to low or undetectable levels is the optimal treatment response. Several studies in HBeAg-negative population have proposed that discontinuation of antiviral treatment can be considered if undetectable serum HBV DNA is demonstrated on three separate occasions at least 6 months apart. Preliminary data suggests that on-treatment HBsAg quantification may play a role as a predictor of sustained response off-therapy.


Similar content being viewed by others
Abbreviations
- (CHB):
-
chronic hepatitis B
- (HBeAg):
-
hepatitis B e antigen
- (HCC):
-
hepatocellular carcinoma
- (ccc DNA):
-
covalently closed circular DNA
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wiersma S. The global burden of disease of viral hepatitis. Viral Hepat. 2011;19:9–10.
Lavanchy D. Hepatitis B, virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11(2):97–107.
Beasley RP. Hepatitis B, virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61(10):1942–56.
Fattovich G. Natural history and prognosis of hepatitis B. Semin Liver Dis. 2003;23:47–58.
McMahon BJ. The natural history of chronic hepatitis B virus infection. Semin Liver Dis. 2004;24:17–21.
Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127 Suppl. 1:S35–50.
Iloeje UH, Yang HI, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Risk evaluation of viral load elevation and associated liver disease/cancer-In HBV (the REVEAL-HBV) study group. Gastroenterology. 2006;130(3):678–86.
Chen C-J, Yang H, Su J, et al. for the REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73.
Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2(8359):1099–102.
McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151(4):599–60.
Coursaget P, Yvonnet B, Chotard J, et al. Age- and sex-related study of hepatitis B virus chronic carrier state in infants from an endemic area (Senegal). J Med Virol. 1987;22(1):1–5.
Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2001;34:617–24.
Brunetto MR, Giarin M, Oliveri F, et al. ‘e’ Antigen defective hepatitis B virus and course of chronic infection. J Hepatol. 1991;13:S82–6.
Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000–summary of a workshop. Gastroenterology. 2001;120:1828–53.
Hadziyannis SJ, Papatheodoridis GV. Hepatitis Be antigen negative chronic hepatitis B – natural history and treatment. Semin Liver Dis. 2006;26:130–41.
Yoo BC, Park JW, Kim HJ, et al. Precore and core promoter mutations of hepatitis B virus and hepatitis B e antigen negative chronic hepatitis B in Korea. J Hepatol. 2003;38:98–103.
Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–31.
Papatheodoridis GV, Dimou E, Dimakopoulos K, Manolakopoulos S, Rapti I, Kitis G, et al. Outcome of hepatitis B e antigen-negative chronic hepatitis B on long-term nucleos(t)ide analog therapy starting with lamivudine. Hepatology. 2005;42:121–9.
Lee HW, Lee HJ, Hwang JS, et al. Lamivudine maintenance beyond one year after HBeAg seroconversion is a major factor for sustained virologic response in HBeAg-positive chronic hepatitis B. Hepatology. 2010;51:415–21.
Yeh CT, Hsu CW, Chen YC, et al. Withdrawal of lamivudine in HBeAg-positive chronic hepatitis B patients after achieving effective maintained virological suppression. J Clin Virol. 2009;45:114–8.
•• Liaw YF, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012. doi:10.1007/s12072-012-9365-4. Recent update on the recommendations for the optimal management of chronic Hepatitis B. This guideline states that if HBsAg remains positive in HBeAg-negative CHB, cessation of antiviral therapy can be considered after documentation of undetectable HBV-DNA on three separate occasions at least 6 months apart, albeit the 50 % risk of virological relapse within one year after stopping therapy.
Lok AS, McMahon BJ. Chronic hepatitis B: Update 2009. Hepatology. 2009;50:661–2.
•• European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167–85. Recent update on the recommendations for the optimal management of chronic Hepatitis B.
Asselah T, Lada O, Moucari R, et al. Interferon Therapy for Chronic Hepatitis B. Clin Liver Dis. 2007;11:839–49.
Marcellin P, Lau GKK, Bonino F, et al. Peginterferon alfa-2 alone, lamivudine alone and the two in combination combination in patients with HBeAg negative chronic hepatitis B. N Engl J Med. 2004;351:1206–17.
Marcellin P, Bonino F, Lau GK, et al. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology. 2009;136:2169–79.
Chan HL, Wang H, Niu J, et al. Two-year lamivudine treatment for hepatitis B e antigen-negative chronic hepatitis B: a double-blind, placebo-controlled trial. Antivir Ther. 2007;12:345–53.
Fung SK, Wong F, Hussain M, et al. Sustained response after a 2-year course of lamivudine treatment of hepatitis e antigen negative chronic hepatitis B. J Viral Hepat. 2004;11:432–8.
Chien RN, Liaw YF. Short-term lamivudine therapy in HBeAg negative chronic active hepatitis B in Taiwan. Antivir Ther. 2006;11:947–52.
Liaw YF, Leung N, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatits B: a 2008 update. Hepatol Int. 2008;2:263–83.
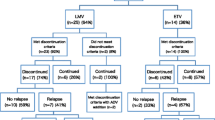
• Liu F, Wang L, Li XY, et al. Poor durability of lamivudine effectiveness despite stringent cessation criteria: a prospective clinical study in hepatitis B e antigen-negative chronic hepatitis B patients. J Gastroenterol Hepatol. 2011;26:456–60. Cessation of lamivudine treatment resulted in cumulative relapse rates of 44 % at 12 months and increasing to 56 % at 60 months post-treatment. Younger age < 20 years was the only predictive factor for virological relapse.
• Liang Y, Jiang J, Su M, et al. Predictors of relapse in chronic hepatitis B after discontinuation of antiviral therapy. Aliment Pharmacol Ther. 2011;34:344–52. Virological relapse, defined as HBV DNA >1000 copies/mL, occurred in 47 % (20/43) of HBeAg-negative CHB patients after discontinuation of antiviral therapy according to the APASL guidelines. Presence of pre-existing lamivudine resistance, delayed suppression of HBV DNA to undetectable level during antiviral therapy and higher HBsAg level at the end of treatment were independent predictors of virological relapse.
•• Hadziyannis SJ, Sevastianos V, Rapti I, et al. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology. 2012;143:629–36. Discontinuation of adefovir after 4 or 5 years may result in sustained response with HBVDNA < 2000 IU/mL in more than 50 % with nearly 40 % of patients achieved HBsAg clearance. Higher pretreatment and end of treatment ALT levels, lower HBsAg levels at the end of treatment and no re-treatment with antivirals after discontinuation of adefovir therapy were associated with clearance of HBsAg on multivariate analysis.
Manesis EK, Papatheodoridis GV, Tiniakos DG, et al. Hepatitis B surface antigen: relation to hepatitis B replication parameters in HBeAg-negative chronic hepatitis B. J Hepatol. 2011;55:61–8.
Werle-Lapostolle B, Bowden S, Locarnini S, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–8.
Volz T, Lutgehetmann M, Wachtler P, et al. Impaired intrahepatic hepatitis B virus productivity contributes to low viremia in most HBeAg-negative patients. Gastroenterology. 2007;133:843–52.
Thompson AJ, Nguyen T, Iser D, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51:1933–44.
Moucari R, Mackiewicz V, Lada O, et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009;49:1151–7.
Manesis EK, Hadziyannis ES, Angelopoulou OP, et al. Prediction of treatment-related HBsAg loss in HBeAg-negative chronic hepatitis B: a clue from serum HBsAg levels. Antivir Ther. 2007;12:73–82.
Borgniet O, Parvaz P, Bouix C, et al. Clearance of serum HBsAg and anti-HBs seroconversion following antiviral therapy for chronic hepatitis B. J Med Virol. 2009;81:1336–42.
Zoutendijk R, Hansen BE, van Vuuren AJ, et al. Serum HBsAg decline during long-term potent nucleos(t)ide analogue therapy for chronic hepatitis B and prediction of HBsAg loss. J Infect Dis. 2011;204:415–8.
•• Chan HL, Wong GL, Chim AM, et al. Prediction of off-treatment response to lamivudine by serum hepatitis B surface antigen quantification in hepatitis B e antigen-negative patients. Antivir Ther. 2011;16:1249–57. Cessation of antiviral treatment may be considered if HBsAg levels reduce by > 1 log IU/ml from baseline to ≤ 200 IU/ml with minimal risk of virological relapse post-treatment discontinuation.
Conflict of Interest
Weng Kai Chan declares that he has no conflict of interest.
Soek-Siam Tan declares that she has no conflict of interest.
Rosmawati Mohamed declares that she has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chan, W.K., Tan, SS. & Mohamed, R. Stopping Therapy in HBeAg Negative Disease. Curr Hepatitis Rep 12, 105–111 (2013). https://doi.org/10.1007/s11901-013-0167-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-013-0167-5