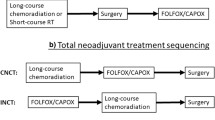
Abstract
New strategies for the treatment of cancer in the rectum should be directed towards the improvement of micrometastatic disease and the reduction of long-term sequelae, without prejudice to good local control. To achieve this, in the last decade, new strategies have been postulated. Treatment with preoperative chemotherapy (CT) alone or induction CT followed by chemoradiation CRT/short course radiation (CRT/SCPRT) or CRT/SCPRT and consolidative CT is being planned. We currently have data from phase II studies with results of stimulating efficacy and/or compliance. New single-arm and randomized trial is underway and will allow us to know the impact on survival outcomes and long-term sequelae of these strategies.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gastrointestinal Tumour Study Group–GiTSG 7175. Prolongation of disease free interval in surgical treated rectal carcinoma. N Engl J Med. 1985;312:1464–72.
Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–15. 5.
Sauer R, Becker H, Hohenberger W, German Rectal Cancer Study Group, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40.
Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–33.
Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-T4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–25.
Bosset JF, Collette L, Calais G, et al. Chemotherapy with pre-operative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–23 13.
Braendengen M, Tveit KM, Berglund A, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol. 2008;26(22):3687–94.
Stockholm Colorectal Cancer Study Group Randomized study on preoperative radiotherapy in rectal carcinoma. Ann Surg Oncol. 1996;3:423–430.
Anon. Improved survival with preoperative radiotherapy in resectable rectal cancer: Swedish Rectal Cancer Trial. N Engl J Med. 1997;336:980–987.
Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638–46.
Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373(9666):811–20.
Vuong T, Devic S. High-dose-rate pre-operative endorectal brachytherapy for patients with rectal cancer. J Contemp Brachytherapy. 2015;7(2):183–8.
Breugom AJ, Vermeer TA, van den Broek CB, Vuong T, Bastiaannet E, Azoulay L, et al. Effect of preoperative treatment strategies on the outcome of patients with clinical T3, non-metastasized rectal cancer: a comparison between Dutch and Canadian expert centers. Eur J Surg Oncol. 2015;41(8):1039–44.
Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Pudełko M, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol. 2004;72(1):15–24.
Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomised trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–23.
Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group Trial 01.04. J Clin Oncol. 2012;30(31):3827–33.
•• Bujko K, Wyrwicz L, Rutkowski A, Malinowska M, Pietrzak L, Kryński J, et al. Polish Colorectal Study Group. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27(5):834–42. Very important Randomised trial examining short course preoperative radiotherapy (SCPRT) followed by FOLFOX and long course chemoradiation (CRT). There were no differences were observed in local efficacy (pCR and R0 resection rate) between 5 × 5 Gy with consolidation FOLFOX chemotherapy versus long-course chemoradiation.But, an improved overall survival and lower acute toxicity favoured the 5 × 5 Gy schedule with FOLFOX consolidation chemotherapy.
• Ansari N, Solomon MJ, Fisher RJ, et al., Acute adverse events and postoperative complications in a randomized trial of preoperative short-course radiotherapy versus long-course chemoradiotherapy for T3 Adenocarcinoma of the Rectum: Trans-Tasman Radiation Oncology Group Trial (TROG 01.04). Ann Surg. 2016. Randomised trial examining short course preoperative radiotherapy (SCPRT) and long course chemoradiation (CRT). There were significantly less AEs for SCPRT compared with CRT but no statistically significant differences in postoperative surgical complications.
Van Gijn W, Marijnen C, Nagtegaal I, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–82.
Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184–90.
Glynne-Jones R, Counsell N, Quirke P, et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. 2014;25:1356–62.
Sainato A, Cernusco Luna Lunzia V, Valentini V, et al. No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Oncol. 2014;113:223–9.
Breugom AJ, van Gijn W, Muller EW, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomised phase III trial. Ann Oncol. 2015;26:696–701.
Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679–87.
Hong Y, Nam B, Kim K, et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2014;15:1245–53.
Marijnen C, Van de Velde C, Putter H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2005;23:1847–58.
MERCURY. Study Group: Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333:779.
Chang GJ, Agarwal A, Maru DM, et al. Histopathologic responses in primary tumors of patients with colorectal carcinoma after neoadjuvant systemic chemotherapy alone. European Society of Medical Oncology 12th World Con- gress on Gastrointestinal Cancer, Barcelona, España, June 30-July 3, 2010 (abstr P-0032).
•• Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513–8. First trial demonstrating encouraging local activity with high PCR rate with systemic chemotherapy only (no CRT) in intermediate-risk rectal cancer.
• Fernandez-Martos C, Brown G, Estevan R, et al. Preoperative chemotherapy in patients with intermediate-risk rectal adenocarcinoma selected by high-resolution magnetic resonance imaging: the GEMCAD 0801 Phase II Multicenter Trial. Oncologist. 2014;19(10):1042–3. First phase II multicentric trial with MRI selected high/intermediate-risk patients, confirming local activity of neoadjuvant systemic chemotherapy treatment.
Patel U, Brown G, Machado I, et al. MRI assessment and outcomes in patients receiving neoadjuvant chemotherapy only for Primary Rectal Cancer: longterm results from the GEMCAD 0801 trial. Ann Oncol. In press.
Uehara K, Hiramatsu K, Maeda A, et al. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: NSOG 03 phase II trial. Jpn J Clin Oncol. 2013;43(10):964–71.
Hasegawa J, Nishimura J, Mizushima T, et al. Neoadjuvant capecitabine and oxaliplatin (XELOX) combined with bevacizumab for high-risk localized rectal cancer. Cancer Chemother Pharmacol. 2014;73(5):1079–87.
Ishii Y, Hasegawa H, Endo T, et al. Medium-term results of neoadjuvant systemic chemotherapy using irinotecan, 5-fluorouracil, and leucovorin in patients with locally advanced rectal cancer. Eur J Surg Oncol. 2010;36(11):1061–5.
• Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J Clin Oncol. 2016;34(27):3300–7. This randomised trial compares neoadjuvant chemotherapy alone versus two CRT regimes (with and without oxaliplatin) in cT3/T4 patients. Preliminary results showed better local efficacy parameters in the combined modality arms but with similar R0 resection rates and more toxicity.
Chand M, Bhangu A, Wotherspoon A, et al. EMVI-positive stage II rectal cancer has similar clinical outcomes as stage III disease following pre-operative chemoradiotherapy. Ann Oncol. 2014;25:858–63.
Bhangu A, Fitzgerald JE, Slesser A, et al. Prognostic significance of extramural vascular invasion in T4 rectal cancer. Color Dis. 2013;15(11).
Glynne-Jones R, Grainger J, Harrison J, et al. Neoadjuvant chemotherapy prior to preoperative chemoradiation or radiation in rectal cancer: should we be more cautious? Br J Cancer. 2006;94:363–71.
Taylor F, Quirke P, Heald R, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY Study. J Clin Oncol. 2013;32:34–43.
• Chua Y, Barbachano Y, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:241–48. This phase II trial demonstrated that the strategy of induction with chemotherapy at therapeutic doses prior to CRT and surgery was feasible with good activity.
•• Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiation followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiation and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo Cancer de Recto 3 Study. J Clin Oncol. 2010;28(5):859–65. Phase II randomized trial demonstrating better compliance and less toxicity of chemotherapy when given before than after CRT and surgery without compromise of local control.
Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol. 2015;26(8):1722–28.
Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol. 2012;30(14):1620–7.
Marechal R, Vos B, Polus M, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. 2012;23:1525–30.
Nogue M, Salud A, Vicente P, et al. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging–defined poor-prognosis locally advanced rectal cancer: the AVACROSS Study. Oncologist. 2011;16:614–20.
Schou JV, Larsen FO, Rasch L, et al. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol. 2012;23:2627–33.
Cercek A, Goodman KA, Hajj C, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Cancer Netw. 2014;12:513–9.
Perez K, Safran H, Sikov W, et al. Complete neoadjuvant treatment for rectal cancer: the Brown University Oncology Group CONTRE Study. Am J Clin Oncol. 2014.
Sclafani F, Gonzalez D, Cunningham D, et al. TP53 mutational status and cetuximab benefit in rectal cancer: 5-year results of the EXPERT-C trial. J Natl Cancer Inst. 2014;106(7).
Gollins S, Sebag-Montefiore D, Adams R, et al. A phase II single arm feasibility trial of neoadjuvant chemotherapy (NAC) with oxaliplatin/fluorouracil (OxMdG) then short-course preoperative radiotherapy (SCPRT) then immediate surgery in operable rectal cancer (ORC): COPERNICUS (NCT01263171). J Clin Oncol. 2015;33(suppl; abstr 3609).
Bujko K, Michalski W, Kepka L, Nowacki MP, Nasierowska-Guttmejer A, Tokar P, et al. Association between pathologic response in metastatic lymph nodes after preoperative chemoradiotherapy and risk of distant metastases in rectal cancer: an analysis of outcomes in a randomized trial. Int J Radiat Oncol Biol Phys. 2007;67(2):369–77.
Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–96.
Yeo SG, Oh JH, Kim DY, Baek JY, Kim SY, Park JW, et al. Preoperative short-course concurrent chemoradiation therapy followed by delayed surgery for locally advanced rectal cancer: a phase 2 multicenter study (KROG 10-01). Int J Radiat Oncol Biol Phys. 2013;86(1):3.
Myerson RJ, Tan B, Hunt S, Olsen J, Birnbaum E, Fleshman J, et al. Five fractions of radiation therapy followed by 4 cycles of FOLFOX chemotherapy as preoperative treatment for rectal cancer. Int J Radiat Oncol Biol Phys. 2014;88(4):829–36.
Nilsson PJ, van Etten B, Hospers GA, Påhlman L, van de Velde CJ, Beets-Tan RG, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer—the RAPIDO trial. BMC Cancer. 2013;13:279. doi:10.1186/1471-2407-13-279.
National Clinical Practice Guidelines in Oncology (NCCN Guidelines): Rectal Cancer Version 2.2017 — December 22, 2016 www.ncrn.org. (last accessed 01/01/2017).
Gerard JP, Azria D, Gourgou-Bourgade S, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30(36):4558–65.
Hernando-Requejo O, López M, Cubillo A, et al. Complete pathological responses in locally advanced rectal cancer after preoperative IMRT and integrated-boost chemoradiation. Strahlenther Onkol. 2014;190(6):515–20.
Engels B, Tournel K, Everaert H, et al. Phase II study of preoperative helical tomotherapy with a simultaneous integrated boost for rectal cancer. Int J Radiat Oncol Biol Phys. 2012;83(1):142–8.
Appelt AL, Pløen J, Vogelius IR, Bentzen SM, Jakobsen A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(1):74–80. 35.36.
Burbach JP, den Harder AM, Intven M, van Vulpen M, Verkooijen HM, Reerink O. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol. 2014;113(1):1.
Jakobsen A, Ploen J, Vuong T, Appelt A, Lindebjerg J, Rafaelsen SR. Dose-effect relationship in chemoradiotherapy for locally advanced rectal cancer: a randomized trial comparing two radiation doses. Int J Radiat Oncol Biol Phys. 2012;84(4):949–5.
Appelt AL, Vogelius IR, Pløen J, Rafaelsen SR, Lindebjerg J, Havelund BM, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16(8):919–27.
Bosset JF, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results--EORTC 22921. J Clin Oncol. 2005;23(24):5620–7.
Hoffheinz RD, Wenz F, Post S. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13(6):579–88.
O’Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014;32(18):1927–34.
Navarro M, Dotor E, Rivera F, et al. A Phase II study of preoperative radiotherapy and concomitant weekly irinotecan in combination with protracted venous infusion 5-fluorouracil, for resectable locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66(1):201–5.
Gollins S, Sun Myint A, Haylock B, et al. Preoperative chemoradiotherapy using concurrent capecitabine and irinotecan in magnetic resonance imaging-defined locally advanced rectal cancer: impact on long-term clinical outcome. J Clin Oncol. 2011;29(8):1042–9.
Mohiuddin M, Paulus R, Mitchell E, Hanna N, Yuen A, Nichols R, et al. Neoadjuvant chemoradiation for distal rectal cancer: 5-year updated results of a randomized phase 2 study of neoadjuvant combined modality chemoradiation for distal rectal cancer. Int J Radiat Oncol Biol Phys. 2013;86(3):523–8.
Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29(20):2773–80.
Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28(10):1638–44.
Schmoll H-J, Haustermans K, Price TJ, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: first results of the PETACC-6 randomized trial (abstract). J Clin Oncol. 2013; 31(suppl; abstr 3531).
Allegra CJ, Yothers G, O’Conelll MJ, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: a phase III randomized clinical trial. J Natl Cancer Inst. 2015;107(11).
Jiao D, Zhang R, Gong Z, Liu F, Chen Y, Yu Q, et al. Fluorouracil-based preoperative chemoradiotherapy with or without oxaliplatin for stage II/III rectal cancer: a 3-year follow-up study. Chin J Cancer Res. 2015;27:588–96.
Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, et al. German Rectal Cancer Study Group. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979–89.
Yang YJ, Cao L, Li ZW, Zhao L, Wu HF, Yue D, et al. Fluorouracil-based neoadjuvant chemoradiotherapy with or without oxaliplatin for treatment of locally advanced rectal cancer: an updated systematic review and meta-analysis. Oncotarget. 2016;7(29):45513–24.
Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Timing of rectal cancer response to chemoradiation consortium. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):957–66.
Radu C, Berglund Å, Påhlman L, Glimelius B. Short course preoperative radiotherapy with delayed surgery in rectal cancer—a retrospective study. Radiother Oncol. 2008;13:343–9.
Hatfield P, Hingorani M, Radhakrishna G, Cooper R, Melcher A, Crellin A, et al. Short-course radiotherapy, with elective delay prior to surgery, in patients with unresectable rectal cancer who have poor performance status or significant co-morbidity. Radiother Oncol. 2009;13:210–4.
Pettersson D, Holm T, Iversen H, Blomqvist L, Glimelius B, Martling A. Preoperative short-course radiotherapy with delayed surgery in primary rectal cancer. Br J Surg. 2012;13:577–83.
van Dijk TH, Tamas K, Beukema JC, Beets GL, Gelderblom AJ, de Jong KP, et al. Evaluation of short-course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann Oncol. 2013;24(7):1762–9.
Michael M, Chander S, McKendrick J, MacKay JR, Steel M, Hicks R. Phase II trial evaluating the feasibility of interdigitating folfox with chemoradiotherapy in locally advanced and metastatic rectal cancer. Br J Cancer. 2014;111(10):1924–31.
De Ruysscher D, Pijls-Johannes MM, Bentzen S, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol. 2006;24(7):1053–63.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Carlos Fernandez-Martos and Alfonso Garcia-Fadrique declare that they have no conflict of interest. Rob Glynne-Jones has received research funding through grants from Roche and Merck Serono, has received speaker’s honoraria from Roche, Amgen, Servier, Sanofi and Merck Serono and has received compensation from Eli Lilly, Roche, Home Nutrition, Servier, Sanofi, Eisai and Amgen for service on advisory boards.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Radiation Therapy and Radiation Therapy Innovations in Colorectal Cancer
Rights and permissions
About this article
Cite this article
Fernandez-Martos, C., Fadrique, A.G. & Glynne-Jones, R. Optimal Sequencing of Neoadjuvant Therapies (NAT) in Rectal Cancer: Upfront Chemotherapy vs. Upfront Chemoradiation. Curr Colorectal Cancer Rep 13, 154–164 (2017). https://doi.org/10.1007/s11888-017-0358-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-017-0358-5