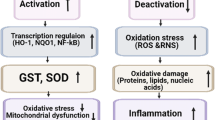
Abstract
As atherosclerosis is still one of the major causes of death in Western populations, it is important to identify those individuals who are at increased risk for the disease so that aggressive treatment may be administered as early as possible. Following the understanding that oxidative stress has a pivotal role in the development and progression of atherosclerosis, many polymorphisms in genes that are related to redox systems were examined for their association with increased risk for cardiovascular disease (CVD). Although many polymorphisms were studied, only a handful showed consistent relevance to CVD in different trials. This article focuses on six of these polymorphisms, examining their effect on the risk for CVD as well as their effect on protein expression and function. Reports regarding pharmacogenetic implications of these polymorphisms, where such exist, are discussed as well.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104(4):503–16.
Kondo T, Hirose M, Kageyama K. Roles of oxidative stress and redox regulation in atherosclerosis. J Atheroscler Thromb. 2009;16(5):532–8.
Farbstein D, Levy AP. The genetics of vascular complications in diabetes mellitus. Cardiol Clin. 2010;28(3):477–96.
Marsden PA, Heng HH, Scherer SW, Stewart RJ, Hall AV, Shi XM, et al. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem. 1993;268(23):17478–88.
Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75(2):247–60.
Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43(3):521–31.
Napoli C, Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009;32(8):1103–8.
Napoli C, Ignarro LJ. Polymorphisms in endothelial nitric oxide synthase and carotid artery atherosclerosis. J Clin Pathol. 2007;60(4):341–4.
McDonald DM, Alp NJ, Channon KM. Functional comparison of the endothelial nitric oxide synthase Glu298Asp polymorphic variants in human endothelial cells. Pharmacogenetics. 2004;14(12):831–9.
Naber CK, Baumgart D, Altmann C, Siffert W, Erbel R, Heusch G. eNOS 894 T allele and coronary blood flow at rest and during adenosine-induced hyperemia. Am J Physiol. 2001;281(5):H1908–1912.
Rossi GP, Taddei S, Virdis A, Cavallin M, Ghiadoni L, Favilla S, et al. The T-786 C and Glu298Asp polymorphisms of the endothelial nitric oxide gene affect the forearm blood flow responses of Caucasian hypertensive patients. J Am Coll Cardiol. 2003;41(6):938–45.
Gulec S, Karabulut H, Ozdemir AO, Ozdol C, Turhan S, Altin T, et al. Glu298Asp polymorphism of the eNOS gene is associated with coronary collateral development. Atherosclerosis. 2008;198(2):354–9.
Miyamoto Y, Saito Y, Nakayama M, Shimasaki Y, Yoshimura T, Yoshimura M, et al. Replication protein A1 reduces transcription of the endothelial nitric oxide synthase gene containing a -786 T– > C mutation associated with coronary spastic angina. Hum Mol Genet. 2000;9(18):2629–37.
Wang XL, Sim AS, Wang MX, Murrell GA, Trudinger B, Wang J. Genotype dependent and cigarette specific effects on endothelial nitric oxide synthase gene expression and enzyme activity. FEBS Lett. 2000;471(1):45–50.
Jeerooburkhan N, Jones LC, Bujac S, Cooper JA, Miller GJ, Vallance P, et al. Genetic and environmental determinants of plasma nitrogen oxides and risk of ischemic heart disease. Hypertension. 2001;38(5):1054–61.
Li J, Wu X, Li X, Feng G, He L, Shi Y. The endothelial nitric oxide synthase gene is associated with coronary artery disease: a meta-analysis. Cardiology. 2010;116(4):271–8.
Casas JP, Cavalleri GL, Bautista LE, Smeeth L, Humphries SE, Hingorani AD. Endothelial nitric oxide synthase gene polymorphisms and cardiovascular disease: a HuGE review. Am J Epidemiol. 2006;164(10):921–35.
Comhair SA, Erzurum SC. The regulation and role of extracellular glutathione peroxidase. Antioxid Redox Signal. 2005;7(1–2):72–9.
• Margis R, Dunand C, Teixeira FK, Margis-Pinheiro M: Glutathione peroxidase family - an evolutionary overview. FEBS J 2008, 275(15):3959–3970. This is an excellent review of the glutathione peroxidase family of proteins and their functions.
Voetsch B, Loscalzo J. Genetic determinants of arterial thrombosis. Arterioscler Thromb Vasc Biol. 2004;24(2):216–29.
Voetsch B, Jin RC, Bierl C, Benke KS, Kenet G, Simioni P, et al. Promoter polymorphisms in the plasma glutathione peroxidase (GPx-3) gene: a novel risk factor for arterial ischemic stroke among young adults and children. Stroke. 2007;38(1):41–9.
Kenet G, Freedman J, Shenkman B, Regina E, Brok-Simoni F, Holzman F, et al. Plasma glutathione peroxidase deficiency and platelet insensitivity to nitric oxide in children with familial stroke. Arterioscler Thromb Vasc Biol. 1999;19(8):2017–23.
• Voetsch B, Jin RC, Bierl C, Deus-Silva L, Camargo EC, Annichino-Bizacchi JM, Handy DE, Loscalzo J: Role of promoter polymorphisms in the plasma glutathione peroxidase (GPx-3) gene as a risk factor for cerebral venous thrombosis. Stroke 2008, 39(2):303–307. This article demonstrates the importance of glutathione peroxidase polymorphism in stroke risk.
Lei XG, Cheng WH, McClung JP. Metabolic regulation and function of glutathione peroxidase-1. Annu Rev Nutr. 2007;27:41–61.
Hamanishi T, Furuta H, Kato H, Doi A, Tamai M, Shimomura H, et al. Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in japanese type 2 diabetic patients. Diabetes. 2004;53(9):2455–60.
Nemoto M, Nishimura R, Sasaki T, Hiki Y, Miyashita Y, Nishioka M, et al. Genetic association of glutathione peroxidase-1 with coronary artery calcification in type 2 diabetes: a case control study with multi-slice computed tomography. Cardiovasc Diabetol. 2007;6:23.
Winter JP, Gong Y, Grant PJ, Wild CP. Glutathione peroxidase 1 genotype is associated with an increased risk of coronary artery disease. Coron Artery Dis. 2003;14(2):149–53.
Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349(17):1605–13.
Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1(5):228–37.
Jakubowski H. The pathophysiological hypothesis of homocysteine thiolactone-mediated vascular disease. J Physiol Pharmacol. 2008;59 Suppl 9:155–67.
Obeid R, Herrmann W. Homocysteine and lipids: S-adenosyl methionine as a key intermediate. FEBS Lett. 2009;583(8):1215–25.
Nygard O, Vollset SE, Refsum H, Brattstrom L, Ueland PM. Total homocysteine and cardiovascular disease. J Intern Med. 1999;246(5):425–54.
Kerkeni M, Tnani M, Chuniaud L, Miled A, Maaroufi K, Trivin F. Comparative study on in vitro effects of homocysteine thiolactone and homocysteine on HUVEC cells: evidence for a stronger proapoptotic and proinflammative homocysteine thiolactone. Mol Cell Biochem. 2006;291(1–2):119–26.
Chang PY, Lu SC, Lee CM, Chen YJ, Dugan TA, Huang WH, et al. Homocysteine inhibits arterial endothelial cell growth through transcriptional downregulation of fibroblast growth factor-2 involving G protein and DNA methylation. Circ Res. 2008;102(8):933–41.
Tsai JC, Wang H, Perrella MA, Yoshizumi M, Sibinga NE, Tan LC, et al. Induction of cyclin A gene expression by homocysteine in vascular smooth muscle cells. J Clin Investig. 1996;97(1):146–53.
McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56(1):111–28.
Van Guelpen B, Hultdin J, Johansson I, Witthoft C, Weinehall L, Eliasson M, et al. Plasma folate and total homocysteine levels are associated with the risk of myocardial infarction, independently of each other and of renal function. J Intern Med. 2009;266(2):182–95.
Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. Jama. 2008;299(17):2027–36.
Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–77.
Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369(9576):1876–82.
Lewis SJ, Ebrahim S, Davey Smith G: Meta-analysis of MTHFR 677 C- > T polymorphism and coronary heart disease: does totality of evidence support causal role for homocysteine and preventive potential of folate? BMJ (Clinical research ed 2005, 331(7524):1053.
• Shih DM, Lusis AJ: The roles of PON1 and PON2 in cardiovascular disease and innate immunity. Current opinion in lipidology 2009, 20(4):288–292. This is an excellent review of paraoxonase biology and its relationship to cardiovascular disease.
Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;46(6):1239–47.
Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21(4):473–80.
Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991;286(1–2):152–4.
Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM, et al. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Investig. 1995;96(6):2882–91.
Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394(6690):284–7.
Shih DM, Xia YR, Wang XP, Miller E, Castellani LW, Subbanagounder G, et al. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J Biol Chem. 2000;275(23):17527–35.
Tward A, Xia YR, Wang XP, Shi YS, Park C, Castellani LW, et al. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation. 2002;106(4):484–90.
Deakin S, Leviev I, Brulhart-Meynet MC, James RW. Paraoxonase-1 promoter haplotypes and serum paraoxonase: a predominant role for polymorphic position - 107, implicating the Sp1 transcription factor. Biochem J. 2003;372(Pt 2):643–9.
Wheeler JG, Keavney BD, Watkins H, Collins R, Danesh J. Four paraoxonase gene polymorphisms in 11212 cases of coronary heart disease and 12786 controls: meta-analysis of 43 studies. Lancet. 2004;363(9410):689–95.
Najafi M, Gohari LH, Firoozrai M. Paraoxonase 1 gene promoter polymorphisms are associated with the extent of stenosis in coronary arteries. Thromb Res. 2009;123(3):503–10.
Gaidukov L, Rosenblat M, Aviram M, Tawfik DS. The 192R/Q polymorphs of serum paraoxonase PON1 differ in HDL binding, lipolactonase stimulation, and cholesterol efflux. J Lipid Res. 2006;47(11):2492–502.
• Dahabreh IJ, Kitsios GD, Kent DM, Trikalinos TA: Paraoxonase 1 polymorphisms and ischemic stroke risk: A systematic review and meta-analysis. Genet Med 2010, 12(10):606–615. This article is a recent comprehensive meta-analysis of paraoxonase polymorphisms and their relationship.
Jarvik GP, Hatsukami TS, Carlson C, Richter RJ, Jampsa R, Brophy VH, et al. Paraoxonase activity, but not haplotype utilizing the linkage disequilibrium structure, predicts vascular disease. Arterioscler Thromb Vasc Biol. 2003;23(8):1465–71.
Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. Jama. 2008;299(11):1265–76.
Miao L, St Clair DK. Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med. 2009;47(4):344–56.
Lebovitz RM, Zhang H, Vogel H, Cartwright Jr J, Dionne L, Lu N, et al. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA. 1996;93(18):9782–7.
Jones DA, Prior SL, Tang TS, Bain SC, Hurel SJ, Humphries SE, Stephens JW: Association between the rs4880 superoxide dismutase 2 (C > T) gene variant and coronary heart disease in diabetes mellitus. Diabetes Res Clin Pract 2010.
Mollsten A, Marklund SL, Wessman M, Svensson M, Forsblom C, Parkkonen M, et al. A functional polymorphism in the manganese superoxide dismutase gene and diabetic nephropathy. Diabetes. 2007;56(1):265–9.
Fujimoto H, Kobayashi H, Ogasawara K, Yamakado M, Ohno M. Association of the manganese superoxide dismutase polymorphism with vasospastic angina pectoris. J Cardiol. 2010;55(2):205–10.
Fujimoto H, Taguchi J, Imai Y, Ayabe S, Hashimoto H, Kobayashi H, et al. Manganese superoxide dismutase polymorphism affects the oxidized low-density lipoprotein-induced apoptosis of macrophages and coronary artery disease. Eur Heart J. 2008;29(10):1267–74.
Bowman BH, Kurosky A: Haptoglobin: the evolutionary product of duplication, unequal crossing over, and point mutation. Advances in human genetics 1982, 12:189–261, 453–184.
• Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, Kalet-Litman S, Anbinder Y, Lache O, Nakhoul FM, Asaf R et al: Haptoglobin: Basic and Clinical Aspects. Antioxidants & redox signaling 2009. This is an excellent summary of work showing basic mechanisms and clinical studies showing the importance of the haptoglobin polymorphism in predicting risk of CVD in diabetes.
Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996;42(10):1589–600.
Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G: Haptoglobin and risk of myocardial infarction, stroke, and congestive heart failure in 342,125 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). Annals of medicine 2009:1–11.
• Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, Alshiek J, Bennett L, Kostenko M, Landau M et al: Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2–2 genotype: a prospective double-blinded clinical trial. Arteriosclerosis, thrombosis, and vascular biology 2008, 28(2):341–347. This prospective, placebo-controlled pharmacogenomic study showed that vitamin E provides significant cardiovascular protection to individuals with diabetes and the haptoglobin 2–2 genotype.
Melamed-Frank M, Lache O, Enav BI, Szafranek T, Levy NS, Ricklis RM, et al. Structure-function analysis of the antioxidant properties of haptoglobin. Blood. 2001;98(13):3693–8.
Asleh R, Marsh S, Shilkrut M, Binah O, Guetta J, Lejbkowicz F, et al. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res. 2003;92(11):1193–200.
Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP. Haptoglobin genotype- and diabetes-dependent differences in iron-mediated oxidative stress in vitro and in vivo. Circ Res. 2005;96(4):435–41.
Timmermann M, Hogger P. Oxidative stress and 8-iso-prostaglandin F(2alpha) induce ectodomain shedding of CD163 and release of tumor necrosis factor-alpha from human monocytes. Free Radic Biol Med. 2005;39(1):98–107.
Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, et al. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol. 2009;174(3):1097–108.
Asleh R, Blum S, Kalet-Litman S, Alshiek J, Miller-Lotan R, Asaf R, et al. Correction of HDL dysfunction in individuals with diabetes and the haptoglobin 2–2 genotype. Diabetes. 2008;57(10):2794–800.
Farbstein D, Levy AP. Pharmacogenomics and the prevention of vascular complications in diabetes mellitus. Therapy. 2009;6(4):531–8.
Pare G, Chasman DI, Parker AN, Zee RR, Malarstig A, Seedorf U, et al. Novel associations of CPS1, MUT, NOX4, and DPEP1 with plasma homocysteine in a healthy population: a genome-wide evaluation of 13 974 participants in the Women’s Genome Health Study. Circ Cardiovasc Genet. 2009;2(2):142–50.
O’Donnell CJ, Cupples LA, D’Agostino RB, Fox CS, Hoffmann U, Hwang SJ, Ingellson E, Liu C, Murabito JM, Polak JF et al: Genome-wide association study for subclinical atherosclerosis in major arterial territories in the NHLBI’s Framingham Heart Study. BMC Med Genet 2007, 8 Suppl 1:S4.
Acknowledgments
This work was supported by grants from the Israel-US Binational Science Foundation, the Juvenile Diabetes Foundation, and the National Institutes of Health (NIH RO1DK085226).
Disclosure
All of the authors have received grants to their institution from the National Institutes of Health and the Juvenile Diabetes Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farbstein, D., Soloveichik, Y.Z., Levy, N.S. et al. Genetics of Redox Systems and Their Relationship with Cardiovascular Disease. Curr Atheroscler Rep 13, 215–224 (2011). https://doi.org/10.1007/s11883-011-0170-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-011-0170-7