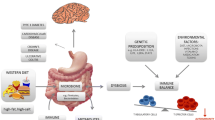
Abstract
Developed societies, although having successfully reduced the burden of infectious disease, constitute an environment where metabolic, cardiovascular, and autoimmune diseases thrive. Living in westernized countries has not fundamentally changed the genetic basis on which these diseases emerge, but has strong impact on lifestyle and pathogen exposure. In particular, nutritional patterns collectively termed the “Western diet”, including high-fat and cholesterol, high-protein, high-sugar, and excess salt intake, as well as frequent consumption of processed and ‘fast foods’, promote obesity, metabolic syndrome, and cardiovascular disease. These factors have also gained high interest as possible promoters of autoimmune diseases. Underlying metabolic and immunologic mechanisms are currently being intensively explored. This review discusses the current knowledge relative to the association of “Western diet” with autoimmunity, and highlights the role of T cells as central players linking dietary influences to autoimmune pathology.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sawcer S et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476(7359):214–9. Comprehensive analysis of genetic risk factors for multiple slerosis.
Cotsapas C, Hafler DA. Immune-mediated disease genetics: the shared basis of pathogenesis. Trends Immunol. 2013;34(1):22–6.
Bogdanos DP et al. Twin studies in autoimmune disease: genetics, gender and environment. J Autoimmun. 2012;38(2–3):J156–69.
Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347(12):911–20.
Okada H et al. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160(1):1–9.
Svenningsson A et al. Incidence of MS during two fifteen-year periods in the Gothenburg region of Sweden. Acta Neurol Scand. 1990;82(3):161–8.
Cook SD et al. Declining incidence of multiple sclerosis in the Orkney Islands. Neurology. 1985;35(4):545–51.
Elhami SR et al. A 20-year incidence trend (1989–2008) and point prevalence (March 20, 2009) of multiple sclerosis in Tehran, Iran: a population-based study. Neuroepidemiology. 2011;36(3):141–7.
Houzen H et al. Increasing prevalence and incidence of multiple sclerosis in northern Japan. Mult Scler. 2008;14(7):887–92.
Kira J. Multiple sclerosis in the Japanese population. Lancet Neurol. 2003;2(2):117–27.
Yamamoto T, Nakahigashi M, Saniabadi AR. Review article: diet and inflammatory bowel disease—epidemiology and treatment. Aliment Pharmacol Ther. 2009;30(2):99–112.
Rook GA. Hygiene hypothesis and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42(1):5–15.
Rapaport B, Karceski S. Multiple sclerosis and stress. Neurology. 2012;79(5):e47–9.
Costenbader KH, Karlson EW. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus. 2006;15(11):737–45.
Hernan MA, Olek MJ, Ascherio A. Cigarette smoking and incidence of multiple sclerosis. Am J Epidemiol. 2001;154(1):69–74.
Brantley PJ, Myers VH, Roy HJ. Environmental and lifestyle influences on obesity. J La State Med Soc. 2005;157(Spec No 1):S19–27.
Landsberg L et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment—a position paper of the The Obesity Society and The American Society of Hypertension. Obesity (Silver Spring). 2013;21(1):8–24.
Procaccini C et al. Obesity and susceptibility to autoimmune diseases. Expert Rev Clin Immunol. 2011;7(3):287–94.
Schwarz S, Leweling H. Multiple sclerosis and nutrition. Mult Scler. 2005;11(1):24–32.
Cashman KD, Shanahan F. Is nutrition an aetiological factor for inflammatory bowel disease? Eur J Gastroenterol Hepatol. 2003;15(6):607–13.
Aho K, Heliovaara M. Risk factors for rheumatoid arthritis. Ann Med. 2004;36(4):242–51.
Andersen V et al. Diet and risk of inflammatory bowel disease. Dig Liver Dis. 2012;44(3):185–94.
Virtanen SM et al. Food consumption and advanced beta cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes: a nested case-control design. Am J Clin Nutr. 2012;95(2):471–8.
Norris JM et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298(12):1420–8.
Agranoff BW, Goldberg D. Diet and the geographical distribution of multiple sclerosis. Lancet. 1974;2(7888):1061–6.
Esparza ML, Sasaki S, Kesteloot H. Nutrition, latitude, and multiple sclerosis mortality: an ecologic study. Am J Epidemiol. 1995;142(7):733–7.
Lauer K. The risk of multiple sclerosis in the U.S.A. in relation to sociogeographic features: a factor-analytic study. J Clin Epidemiol. 1994;47(1):43–8.
Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology. 2009;73(19):1543–50.
Hedstrom AK, Olsson T, Alfredsson L. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult Scler. 2012;18(9):1334–6.
Swank RL et al. Multiple sclerosis in rural Norway its geographic and occupational incidence in relation to nutrition. N Engl J Med. 1952;246(19):722–8.
Ricketts JR, Rothe MJ, Grant-Kels JM. Nutrition and psoriasis. Clin Dermatol. 2010;28(6):615–26.
Naldi L et al. Dietary factors and the risk of psoriasis. Results of an Italian case-control study. Br J Dermatol. 1996;134(1):101–6.
Phillips CM. Nutrigenetics and metabolic disease: current status and implications for personalised nutrition. Nutrients. 2013;5(1):32–57.
Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev. 2006;27(7):750–61.
Ono T, Guthold R, Strong K. WHO Global Comparable Estimates. https://apps.who.int/infobase/; 2005.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7.
Sterry W, Strober BE, Menter A. Obesity in psoriasis: the metabolic, clinical and therapeutic implications. Report of an interdisciplinary conference and review. Br J Dermatol. 2007;157(4):649–55.
Ferraz-Amaro I et al. Metabolic syndrome in rheumatoid arthritis. Mediat Inflamm. 2013;2013:710928.
Chung CP et al. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis. 2008;196(2):756–63.
Mijac DD et al. Nutritional status in patients with active inflammatory bowel disease: prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med. 2010;21(4):315–9.
Delgado-Aros S et al. Obesity is associated with increased risk of gastrointestinal symptoms: a population-based study. Am J Gastroenterol. 2004;99(9):1801–6.
Desreumaux P et al. Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology. 1999;117(1):73–81.
Ouchi N et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97.
Winer S et al. Obesity predisposes to Th17 bias. Eur J Immunol. 2009;39(9):2629–35.
Poutahidis T et al. Microbial reprogramming inhibits Western diet-associated obesity. PLoS ONE. 2013;8(7):e68596. Study demonstrating that probiotic bacteria can prevent obesity in a Treg-dependent manner.
Cipolletta D et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486(7404):549–53.
Cipolletta D et al. Tissular T(regs): a unique population of adipose-tissue-resident Foxp3+CD4+ T cells that impacts organismal metabolism. Semin Immunol. 2011;23(6):431–7. Comprehensive review on fat-residing Tregs.
Sumarac-Dumanovic M et al. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond). 2009;33(1):151–6.
Ahmed M, Gaffen SL. IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev. 2010;21(6):449–53.
Paik J et al. High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible Mdr1a-/- male mice. J Nutr. 2013;143(8):1240–7.
Jhun JY et al. Obesity aggravates the joint inflammation in a collagen-induced arthritis model through deviation to Th17 differentiation. Exp Mol Med. 2012;44(7):424–31.
Timmermans S, et al. High fat diet exacerbates neuroinflammation in an animal model of multiple sclerosis by activation of the renin angiotensin system. J NeuroImmune Pharmacol. 2013. doi:10.1007/s11481-013-9502-4.
Sanna V et al. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest. 2003;111(2):241–50.
Piccio L, Stark JL, Cross AH. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J Leukoc Biol. 2008;84(4):940–8.
Unoda K et al. Eicosapentaenoic acid (EPA) induces peroxisome proliferator-activated receptors and ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol. 2013;256(1–2):7–12.
Sanchez-Fidalgo S et al. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. J Nutr Biochem. 2013;24(7):1401–13.
Leslie CA et al. A fish oil diet reduces the severity of collagen induced arthritis after onset of the disease. Clin Exp Immunol. 1988;73(2):328–32.
Aktas O et al. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J Immunol. 2004;173(9):5794–800.
Wu C et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–7. Experimental work on a new salt-sensitive pathway in the control of Th17 responses.
Kleinewietfeld M et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518–22. First study to show an influence of high-salt on human and murine Th17 differentiation and of high-salt diet on EAE severity.
Okada Y, et al. Trans fatty acids exacerbate DSS-induced colitis by promoting the upregulation of macrophage-derived proinflammatory cytokines involved in T helper 17 cell polarization. Clin Exp Immunol. 2013.
Pond CM. Paracrine relationships between adipose and lymphoid tissues: implications for the mechanism of HIV-associated adipose redistribution syndrome. Trends Immunol. 2003;24(1):13–8.
Matarese G et al. Leptin as a metabolic link to multiple sclerosis. Nat Rev Neurol. 2010;6(8):455–61.
De Rosa V et al. Leptin neutralization interferes with pathogenic T cell autoreactivity in autoimmune encephalomyelitis. J Clin Invest. 2006;116(2):447–55.
Brown IJ et al. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38(3):791–813.
Savica V, Bellinghieri G, Kopple JD. The effect of nutrition on blood pressure. Annu Rev Nutr. 2010;30:365–401.
Bragulat E, de la Sierra A. Salt intake, endothelial dysfunction, and salt-sensitive hypertension. J Clin Hypertens (Greenwich). 2002;4(1):41–6.
Guzik TJ et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–60.
Kvakan H et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119(22):2904–12.
Klack K, Bonfa E, Borba Neto EF. Diet and nutritional aspects in systemic lupus erythematosus. Rev Bras Reumatol. 2012;52(3):384–408.
Shapiro L, Dinarello CA. Osmotic regulation of cytokine synthesis in vitro. Proc Natl Acad Sci U S A. 1995;92(26):12230–4.
Junger WG et al. Hypertonic saline enhances cellular immune function. Circ Shock. 1994;42(4):190–6.
Loomis WH et al. Hypertonicity rescues T cells from suppression by trauma-induced anti-inflammatory mediators. Am J Physiol Cell Physiol. 2001;281(3):C840–8.
Go WY et al. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci U S A. 2004;101(29):10673–8.
Kino T et al. Brx mediates the response of lymphocytes to osmotic stress through the activation of NFAT5. Sci Signal. 2009;2(57):ra5.
Woehrle T et al. Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J Leukoc Biol. 2010;88(6):1181–9.
Titze J. Water-free sodium accumulation. Semin Dial. 2009;22(3):253–5.
Machnik A et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15(5):545–52.
Wiig H et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123(7):2803–15. Study demonstrating salt-dependent effects on macrophages in vivo.
Rakova N et al. Long-term space flight simulation reveals infradian rhythmicity in human na(+) balance. Cell Metab. 2013;17(1):125–31. Long-term study on salt intake in humans under highly controlled conditions.
Marchesi J, Shanahan F. The normal intestinal microbiota. Curr Opin Infect Dis. 2007;20(5):508–13.
Kau AL et al. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–36. Comprehensive review on nutrition factors, intestinal microbiota, and immune responses.
Hildebrandt MA, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137(5):1716–24 e1-2.
Hormannsperger G, Haller D. Molecular crosstalk of probiotic bacteria with the intestinal immune system: clinical relevance in the context of inflammatory bowel disease. Int J Med Microbiol. 2010;300(1):63–73.
Maccaferri S, Biagi E, Brigidi P. Metagenomics: key to human gut microbiota. Dig Dis. 2011;29(6):525–30.
Turnbaugh PJ et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4.
Qin J et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60.
Morgan XC et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13(9):R79.
Berer K et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479(7374):538–41. Experimental study linking microbiota to neuroinflammation.
Tlaskalova-Hogenova H et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8(2):110–20.
Lavasani S et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE. 2010;5(2):e9009.
Lee YK et al. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4615–22. Experimental study linking microbiota to neuroinflammation.
Atarashi K et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–41. Study showing the induction of Tregs by specific bacteria.
Atarashi K et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–6. First study showing the induction of functional Tregs by a selected set of human bacteria.
Esplugues E et al. Control of TH17 cells occurs in the small intestine. Nature. 2011;475(7357):514–8. Study on intestinal control of Th17 responses.
Gomez-Vaquero C et al. Nutritional status in patients with rheumatoid arthritis. Joint Bone Spine. 2001;68(5):403–9.
Giugliano D, Esposito K. Mediterranean diet and metabolic diseases. Curr Opin Lipidol. 2008;19(1):63–8.
Torkildsen O et al. Omega-3 fatty acid treatment in multiple sclerosis (OFAMS Study): a randomized, double-blind, placebo-controlled trial. Arch Neurol. 2012;69(8):1044–51.
van Nood E et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–15.
Weinstock JV. Autoimmunity: the worm returns. Nature. 2012;491(7423):183–5.
Acknowledgments
This work was supported by a National MS Society Collaborative Research Center Award CA1061-A-18, National Institutes of Health Grants P01 AI045757, U19 AI046130, U19 AI070352, and P01 AI039671, and by a Jacob Javits Merit award (NS2427) from the National Institute of Neurological Disorders and Stroke, the Penates Foundation and the Nancy Taylor Foundation for Chronic Diseases, Inc. (to David A. Hafler) and by National Institutes of Health Grants P30-ES002109 and U01 CA164337 (to Susan E. Erdman).
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Arndt Manzel, Dominik N. Muller, David A. Hafler, Susan E. Erdman, Ralf A. Linker, and Markus Kleinewietfeld declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Autoimmunity
Rights and permissions
About this article
Cite this article
Manzel, A., Muller, D.N., Hafler, D.A. et al. Role of “Western Diet” in Inflammatory Autoimmune Diseases. Curr Allergy Asthma Rep 14, 404 (2014). https://doi.org/10.1007/s11882-013-0404-6
Published:
DOI: https://doi.org/10.1007/s11882-013-0404-6