Abstract
Pollen cross-allergenicity has practical implications on the management of inhalant allergy, in both evaluation and therapy, especially with allergen vaccine immunotherapy. The study of cross-reactivity among pollen allergens has expanded beyond investigation of crude extracts to the characterization and cloning of numerous pollen proteins. In this review, the interrelationships between these pollen allergens in the context of botanical systematics are examined, to provide a framework for cross-reactivity understanding. Recommendations for choices in evaluation and therapy are given.
Similar content being viewed by others
References and Recommended Reading
Breiteneder H, Radauer C: A classification of plant food allergens. J Allergy Clin Immunol 2004, 113:821–830. This is an excellent current review of several classes of plant allergens that addresses the function of the proteins and, thus, their likelihood of being found in pollen proteins.
Judd WS, Campbell CS, Kellogg EA, Stevens PF: Plant systematics: a phylogenetic approach.Snderland, MA: Sinauer Associates; 1999. This is a superb reference text for those interested in the foundations of present plant systematics.
Weber RW: Cross-reactivity of plant and animal allergens. Clin Rev Allergy Immunol 2001, 21:153–202. This review covers, in great detail, historic studies as well as recent (up to 2001) research on pollen allergen cross-reactivity. Fungal, inhalant animal, and venom allergens are addressed as well.
Schwietz LA, Goetz DW, Whisman BA, Reid MJ: Crossreactivity among conifer pollens. Ann Allergy Asthma Immunol 2000, 84:87–93.
Midoro-Horiuti T, Goldblum RM, Kurosky A, et al.: Molecular cloning of the mountain cedar (Juniperus ashei) pollen major allergen, Jun a 1. J Allergy Clin Immunol 1999, 104:613–617.
Canini A, Giovinazzi J, Iacovacci P, et al.: Localisation of a carbohydrate epitope recognised by human IgE in pollen of Cupressaceae. J Plant Res 2004, 11:147–153.
van Ree R: Carbohydrate epitopes and their relevance for the diagnosis and treatment of allergic diseases. Int Arch Allergy Immunol 2002, 129:189–197.
Tinghino R, Barletta B, Palumbo S, et al.: Molecular characterization of a cross-reactive Juniperus oxycedrus pollen allergen, Jun o 2: a novel calcium-binding allergen. J Allergy Clin Immunol 1998, 101:772–777.
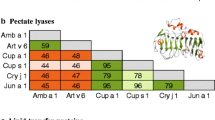
Midoro-Horiuti T, Mathura V, Schein CH, et al.: Major linear IgE epitopes of mountain cedar pollen allergen Jun a 1 map to the pectate lysate catalytic site. Mol Immunol 2003, 40:555–562. This is a fine example of characterization of a major allergen group, providing insight into the reason for its ubiquitous nature within Cupressaceae.
Andersson K, Lidholm J: Characteristics and immunobiology of grass pollen allergens. Int Arch Allergy Immunol 2003, 130:87–107. This paper is a thorough and up-to-date review of the ten defined grass pollen allergen groups. More than any other recent review, this paper provides an excellent reference source for understanding these allergens.
Fahlbusch B, Müller W-D, Rudeschko O, et al.: Detection and quantification of group 4 allergens in grass pollen extracts using monoclonal antibodies. Clin Exp Allergy 1998, 28:799–807.
Grobe K, Becker W-M, Schlaak M, Petersen A: Grass group I allergens (beta-expansins) are novel, papain-related proteinases. Eur J Biochem 1999, 263:33–40.
Weber RW: Patterns of pollen cross-allergenicity. J Allergy Clin Immunol 2003, 112:229–239. This review provides a detailed discussion of recent advances in pollen cross-reactivity.
van Ree R, van Leeuwen WA, van den Berg M, et al.: IgE and IgG cross-reactivity among Lol p I and Lol p II/III: identification of the C-termini of Lol p I, II, and III as cross-reactive structures. Allergy 1994, 49:254–261.
Gavrovic-Jankulovic M, Cirkovic T, Burazer L, et al.: IgE cross-reactivity between meadow fescue pollen and kiwi fruit in patients’ sera with sensitivity to both extracts. J Investig Allergol Clin Immunol 2002, 12:279–286.
Fahlbusch B, Müller W-D, Diener C, Jäger L: Detection of cross reactive determinants in grass pollen extracts using monoclonal antibodies against group IV and group V allergens. Clin Exp Allergy 1993, 23:51–60.
Müller W-D, Karamfilov T, Bufe A, et al.: Group 5 allergens of timothy grass (Phl p 5) bear cross-reacting T cell epitopes with group 1 allergens of rye grass (Lol p 1). Int Arch Allergy Immunol 1996, 109:352–355.
Matthiesen F, Schumacher MJ, Løwenstein H: Characterization of the major allergen of Cynodon dactylon (Bermuda grass) pollen, Cyn d I. J Allergy Clin Immunol 1991, 88:763–774.
Chang ZN, Liu CC, Perng HC, et al.: A common allergic epitope of Bermuda grass pollen shared with other grass pollens. J Biomed Sci 1994, 1:93–99.
Smith PM, Ong EK, Knox RB, Singh MB: Immunological relationships among group I and group V allergens from grass pollens. Mol Immunol 1994, 31:491–498. Research described here helps explain the lack of major crossreactivity between northern and southern grasses.
Tinghino R, Twardosz A, Barletta B, et al.: Molecular, structural, and immunologic relationships between different families of recombinant calcium-binding pollen allergens. J Allergy Clin Immunol 2002, 109:314–320.
Smith PM, Xu H, Swoboda I, Singh MB: Identification of a Ca2+ binding protein as a new Bermuda grass pollen allergen Cyn d 7: IgE cross-reactivity with oilseed rape allergen Bra r 1. Int Arch Allergy Immunol 1997, 114:265–271.
van Ree R, Hoffman DR, van Dijk W, et al.: Lol p XI, a new major grass pollen allergen, is a member of a family of soybean trypsin inhibitor-related proteins. J Allergy Clin Immunol 1995, 95:970–978.
Asturias JA, Arilla MC, Gomez-Bayon N, et al.: Cloning and high level expression of Cynodon dactylon (Bermuda grass) pollen profilin (Cyn d 12) in Escherichia coli: purification and characterization of the allergen. Clin Exp Allergy 1997, 27:1307–1313.
Niederberger V, Laffer S, Froschl R, et al.: IgE antibodies to recombinant pollen allergens (Phl p 1, Phl p 2, Phl p 5, and Bet v 2) account for a high percentage of grass pollen-specific IgE. J Allergy Clin Immunol 1998, 101:258–264.
Wurtzen PA, Nelson HS, Løwenstein H, Ipsen H: Characterization of Chenopodiales (Amaranthus retroflexus, Chenopodium album, Kochia scoparia, Salsola pestifer) pollen allergens. Allergy 1995, 50:489–497.
Barderas R, Villalba M, Lombardero M, Rodriguez R: Identification and characterization of Che a 1 allergen from Chenopodium album pollen. Int Arch Allergy Immunol 2002, 127:47–54.
Kernerman SM, McCullough J, Green J, Ownby DR: Evidence of cross-reactivity between olive, ash, privet, and Russian olive tree pollen allergens. Ann Allergy 1992, 69:493–496.
Bousquet J, Hewitt B, Guerin B, et al.: Allergy in the Mediterranean area. II: Cross-allergenicity among Urticaceae pollens (Parietaria and Urtica). Clin Allergy 1986, 16:57–64.
Miura N: Ramie (Boehmeria nivea) pollen-induced bronchial asthma and allergenic cross-reactivity of ramie and Parietaria. Arerugi 1993, 42:649–655.
Stumvoll S, Westritschnig K, Lidholm J, et al.: Identification of cross-reactive and genuine Parietaria judaica pollen allergens. J Allergy Clin Immunol 2003, 111:974–749.
Asero R, Mistrello G, Roncarolo D, Amato S: Parietaria profilin shows only limited cross-reactivity with birch and grass profilins. Int Arch Allergy Immunol 2003, 133:121–124.
di Somma C, Fiore L, Di Lonardo A, et al.: Cross-reactivity between the major Parietaria allergen and rotovirus VP4 protein. Allergy 2003, 58:503–510. An interesting study reporting the first evidence of cross-reactivity between a pollen allergen and a viral antigen.
Vallverdú A, Asturias JA, Arilla C, et al.: Characterization of recombinant Mercurialis annua major allergen Mer a 1 (profilin). J Allergy Clin Immunol 1998, 101:363–370.
Vallier P, Ballard S, Harf R, et al.: Identification of profilin as an IgE-binding component in latex from Hevea brasiliensis: clinical implications. Clin Exp Allergy 1995, 25:332–339.
Fuchs T, Spitzauer S, Vente C, et al.: Natural latex, grass pollen, and weed pollen share IgE epitopes. J Allergy Clin Immunol 1997, 100:356–364.
Valenta R, Breiteneder H, Pettenberger K, et al.: Homology of the major birch-pollen allergen, Bet v I, with the major pollen allergens of alder, hazel, and hornbeam at the nucleic acid level as determined by cross-hybridization. J Allergy Clin Immunol 1991, 87:677–682.
Niederberger V, Pauli G, Gronlund H, et al.: Recombinant birch pollen allergens (rBet v 1 and rBet v 2) contain most of the IgE epitopes present in birch, alder, hornbeam, hazel, and oak pollen: a quantitative IgE inhibition study with sera from different populations. J Allergy Clin Immunol 1998, 102:579–591.
Bauer L, Ebner C, Hirschwehr R, et al.: IgE cross-reactivity between birch pollen, mugwort pollen and celery is due to at least three distinct cross-reacting allergens: immunoblot investigation of the birch-mugwort-celery syndrome. Clin Exp Allergy 1996, 26:1161–1170.
Seiberler S, Scheiner O, Kraft D, et al.: Characterization of a birch pollen allergen, Bet v III, representing a novel class of Ca2+ binding proteins: specific expression in mature pollen and dependence of patients’ IgE binding on proteinbound Ca2+. EMBO J 1994, 13:3481–3486.
Engel E, Richter K, Obermeyer G, et al.: Immunological and biological properties of Bet v 4, a novel birch pollen allergen with two EF-hand calcium-binding domains. J Biol Chem 1997, 272:28630–28637.
Karamloo F, Schmitz N, Scheuer S, et al.: Molecular cloning and characterization of a birch pollen minor allergen, Bet v 5, belonging to a family of isoflavone reductaserelated proteins. J Allergy Clin Immunol 1999, 104:991–999.
Focke M, Hemmer W, Hayek B, et al.: Identification of allergens in oilseed rape (Brassica napus) pollen. Int Arch Allergy Immunol 1998, 117:105–112.
Hayek B, Vangelista L, Pastore A, et al.: Molecular and immunologic characterization of a highly cross-reactive two EF-hand calcium-binding alder pollen allergen, Aln g 4: structural basis for calcium-modulated IgE recognition. J Immunol 1998, 161:7031–7039.
Neudecker P, Nerkamp J, Eisenmann A, et al.: Solution structure, dynamics, and hydrodynamics of the calcium-bound cross-reactive birch pollen allergen Bet v 4 reveal a canonical monomeric two EF-hand assembly with a regulatory function. J Mol Biol 2004, 336:1141–1157.
Gruehn S, Suphioglu C, O’Hehir R, Volkmann D: Molecular cloning and characterization of hazel pollen protein (70kD) as a luminal binding protein (BiP): a novel cross-reactive plant allergen. Int Arch Allergy Immunol 2003, 131:91–100.
Mittag D, Vieths S, Vogel L, et al.: Soybean allergy in patients allergic to birch pollen: clinical investigation and molecular characterization of allergens. J Allergy Clin Immunol 2004, 113:148–154.
Welch J, Jones MG, Cullinan P, et al.: Sensitization to oilseed rape is not due to cross-reactivity with grass pollen. Clin Exp Allergy 2000, 30:370–375.
Calabozo B, Barber D, Polo F: Studies on the carbohydrate moiety of Pla l 1 allergen: identification of a major N-glycan and significance for the immunoglobulin E-binding activity. Clin Exp Allergy 2002, 32:1628–1634.
Bousquet J, Guérin B, Hewitt B, et al.: Allergy in the Mediterranean area III. Cross reactivity among Oleaceae pollens. Clin Allergy 1985, 15:439–448.
Obispo TM, Melero JA, Carpizo JA, et al.: The main allergen of Olea europaea (Ole e I) is also present in other species of the Oleaceae family. Clin Exp Allergy 1993, 23:311–316.
Martin-Orozco E, Cárdaba B, del Pozo V, et al.: Ole e 1: epitope mapping, cross-reactivity with other Oleaceae pollens and ultrastructural localization. Int Arch Allergy Immunol 1994, 104:160–170.
Batanero E, Villalba M, Monsalve R, Rodriguez R: Crossreactivity between the major allergen from olive pollen and unrelated glycoproteins: evidence of an epitope in the glycan moiety of the allergen. J Allergy Clin Immunol 1996, 97:1264–1271.
Batanero E, Villalba M, Ledesma A, et al.: Ole e 3, an olive-tree allergen, belongs to a widespread family of pollen proteins. Eur J Biochem 1996, 241:772–778.
Ledesma A, González E, Pascual CY, et al.: Are Ca2+-binding motifs involved in the immunoglobulin E-binding of allergens? Olive pollen allergens as model of study. Clin Exp Allergy 2002, 32:1476–1483.
Barral P, Batanero E, Palomares O, et al.: A major allergen from pollen defines a novel family of plant proteins and shows intra- and interspecies cross-reactivity. J Immunol 2004, 172:3644–3651.
Niederberger V, Purohit A, Oster JP, et al.: The allergen profile of ash (Fraxinus excelsior) pollen: cross-reactivity with allergens from various plant species. Clin Exp Allergy 2002, 32:933–941.
Förster-Waldi E, Marchetti M, Schℓl I, et al.: Type I allergy to elderberry (Sambucus nigra) is elicited by a 33.2 kDa allergen with significant homology to ribosomal inactivating proteins. Clin Exp Allergy 2003, 33:1703–1710.
Rafnar T, Griffith IJ, Kuo M-C, et al.: Cloning of Amb a I (Antigen E), the major allergen family of short ragweed pollen. J Biol Chem 1991, 266:1229–1236.
Roebber M, Klapper DG, Marsh DG: Two isoallergens of short ragweed component Ra5. J Immunol 1982, 129:120–125.
Bond JF, Garman RD, Keating KM, et al.: Multiple Amb a I allergens demonstrate specific reactivity with IgE and T cells from ragweed-allergic patients. J Immunol 1991, 146:3380–3385.
Roebber M, Hussain R, Klapper DG, Marsh DG: Isolation and properties of a new short ragweed pollen allergen, Ra6. J Immunol 1983, 131:706–711.
Yunginger JW, Gleich GJ: Mesurement of ragweed antigen E by double antibody radioimmunoassay. J Allergy Clin Immunol 1972, 50:326–337.
Lee YS, Dickinson DB: Characterization of pollen antigens from Ambrosia L. (Compositae) and related taxa by immunoelectrophoresis and radial immunodiffusion. Am J Bot 1979, 66:245–262.
Krilis S, Baldo BA, Raison RL, et al.: Standardization of antigen E in ragweed pollen extracts using a monoclonal antibodybased enzyme immunoassay. J Allergy Clin Immunol 1983, 71:261–265.
Coulter KM, Yang WH, Dorval G, et al.: Specific IgE antibody responses to ragweed allergens Ra5S and Ra5G associated with distinct HLA-DR b genes. Mol Immunol 1987, 24:1207–1210.
Katial RK, Lin FL, Stafford WW, et al.: Mugwort and sage (Artemisia) pollen cross-reactivity: ELISA inhibition and immunoblot evaluation. Ann Allergy Asthma Immunol 1997, 79:340–346.
Brandys J, Grimsøen A, Nilsen BM, et al.: Cross-reactivity between pollen extracts from six Artemisia species. Planta Med 1993, 59:221–228.
Hirschwehr R, Heppner C, Spitzauer S, et al.: Identification of common allergenic structures in mugwort and ragweed pollen. J Allergy Clin Immunol 1998, 101:196–206.
Wopfner N, Willeroidee M, Hebenstreit D, et al.: Molecular and immunological characterization of profilin from mugwort pollen. Biol Chem 2002, 383:1779–1789.
Garcia-Sells FJ, Diaz-Perales A, Snchez-Monge R, et al.:Patterns of reactivity to lipid transfer proteins of plant foods and Artemisia pollen: an in vivo study. Int Arch Allergy Immunol 2002, 128:115–122.
Fernandez C, Martin-Esteban M, Fiandor A, et al.: Analysis of cross-reactivity between sunflower pollen and other pollens of the Composite family. J Allergy Clin Immunol 1993, 92:660–667.
Asturias JA, Arilla MC, Gomez-Bayon N, et al.: Cloning and immunological characterization of the allergen Hel a 2 (profilin) from sunflower pollen. Mol Immunol 1998, 35:469–478.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weber, R.W. Cross-reactivity of pollen allergens. Curr Allergy Asthma Rep 4, 401–408 (2004). https://doi.org/10.1007/s11882-004-0091-4
Issue Date:
DOI: https://doi.org/10.1007/s11882-004-0091-4