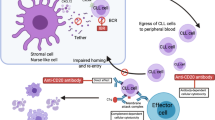
Opinion statement
The standard frontline therapy for diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and mantle cell lymphoma (MCL) includes the use of chemoimmunotherapy and/or radiation therapy. When patients with these diseases relapse or are refractory to therapy, their diseases are considered incurable outside of the setting of an autologous or allogeneic stem cell transplant, which many patients are not candidates for due to age or comorbidities. The oral Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib, targets the B-cell receptor (BCR) signaling pathway that is critical in the survival of these malignancies. It has shown promising activity in certain subtypes of DLBCL, in relapsed or refractory FL, and in relapsed or refractory MCL for which it has recently received FDA approval and should be considered for use in patients in first relapse. Ibrutinib is an oral therapy taken daily that has been well tolerated by patients. Given the high response rates, tolerability, and acceptable toxicities of ibrutinib therapy, it is now being evaluated in combination therapy both in relapsed B-cell malignancies and frontline studies in DLBCL and MCL. Several other promising agents targeting different kinases in the BCR signaling pathway also are currently under investigation.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. Cancer J Clin. 2013;63:11–30.
Mohamed AJ, Yu L, Backesjo CM, et al. Bruton’s tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev. 2009;228:58–73.
A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The non-Hodgkin’s Lymphoma Classification Project. Blood 1997;89:3909-3918.
Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified in gene expression profiling. Nature. 2000;403:503–11.
Persky DO, Unger JM, Spier CM, et al. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited stage aggressive B-cell lymphoma. Southwest Oncology Group study 0014. J Clin Oncol. 2008;26:2258–63.
Horning SJ, Weller E, Kim K, et al. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin’s lymphoma: Easter Cooperative Oncology Group Study 1484. J Clin Oncol. 2004;22:3032–8.
Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B cell lymphoma. N Engl J Med. 2002;346:235–42.
Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26:2717–24.
Wilder RB, Jones D, Tucker SL, et al. Long-term results with radiotherapy for Stage I-II follicular lymphomas. Int J Radiat Oncol Biol Phys. 2001;51:1219–27.
Freidberg JW, Byrtek M, Link BK, et al. Effectiveness of first-line management strategies for stage I follicular lymphoma: analysis of the National LymphoCare Study. J Clin Oncol. 2012;30:3368–75.
Michallet AS, Lebras LL, Bauwens DD, et al. Early stage follicular lymphoma: is there a clinical impact of first-line treatment? [abstract]. Blood 2012;120:Abstract 2722.
The treatment of indolent lymphomas: watchful waiting v aggressive combined modality treatment. Semin Hematol 1988;25:11–16.
Ardeshna KM, Smith P, Norton A, et al. Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: a randomised controlled trial. Lancet. 2003;362:516–22.
Ardeshna K, Qian W, Smith P, et al. An Intergroup randomized trial of rituximab versus a watch and wait strategy in patients with stage II, III, IV asymptomatic, non-bulky follicular lymphoma (grades 1, 2, 3a). A preliminary analysis [abstract] Blood 2010;116:Abstract 6.
Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–32.
Marcu R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26:4579–86.
Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab (B-R) versus CHOP plus rituximab (CHOP-R) as first-line treatment in patients with indolent and mantle cell lymphomas (MCL): an open-label, multicentre, randomized, phase 3 non-inferiority trial. Lancet. 2013;381:1203–10.
Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumor burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42–51.
Howard OM, Gribben JG, Neuberg DS, Grossbard M, Poor C, Janicek MJ, et al. Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: molecular complete responses are not predictive of progression-free survival. J Clin Oncol. 2002;20(5):1288–94.
Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progress-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–84.
Hiddemann W, Unterhalt M, Dreyling M, Hossfeld D, Lengfelder E, Metzner B, et al. The addition of Rituximab (R) to combination chemotherapy (CT) significantly improves the treatment of mantle cell lymphoma (MCL): results of two prospective randomized studies by the German Low Grade Lymphoma Study Group (GLSG). Blood. 2002;100.
Romaguera J, Fayad L, Rodriquez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23:7013–23.
Bernstein SH, Epner E, Unger JM, et al. A phase II multicenter trial of hyperCVAD MTX/Ara-Cand rituximab in patients with previously untreated mantle cell lymphoma; SWOG 0213. Ann Oncol. 2013;24:1587–93.
Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized Phase II multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–93.
Damon LE, Johnson JL, Niedzwiecki D, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27:6101–8.
Delarue R, Haioun C, Ribrag V, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation (ASCT) in mantle cell lymphoma (MCL): a phase II study from the GELA. Blood. 2013;121:48–53.
Kluin-Nelemans JC, Hoster E, Walewski J, et al. R-CHOP versus R-FC followed by maintenance with rituximab versus interferon-alfa: outcome of the first randomized trial for elderly patients with mantle cell lymphoma [abstract]. Blood 2011;118:Abstract 439.
Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–5.
Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;27:4284–90.
Moccia AA, Hitz F, Hoskins P, et al. Gemcitabine, dexamethasone, and cisplatin (GDP) is an effective and well-tolerated outpatient salvage therapy for relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and Hodgkin lymphoma (HL). Blood. 2012;116:113.
Velasquez WS, McLaughlin P, Tucker S, et al. ESHAP-an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994;12:1169–76.
Rummel MJ, Al-Batran SE, Kim SZ, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:3383–9.
Robinson KS, Williams ME, van der Jagt RH, Cohen P, Herst JA, Tulpule A, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:4473–9.
Wiernik PH, Lossos IS, Tuscano JM, Justice G, Vose JM, Cole CE, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:4952–7.
Witzig TE, Wiernik PH, Moore T, Reeder C, Cole C, Justice G, et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin's lymphoma. J Clin Oncol. 2009;27:5404–9.
Leonard J, Jung SH, Johnson JL, et al. CALGB 50401: a randomized trial of lenalidomide alone verus lenalidomide plus rituximab in patients with recurrent follicular lymphoma [abstract]. J Clin Oncol 2012;30:Abstract 8000.
Zinzani PL, Vose JM, Czuczman MS, et al. Phase II multicenter study of the safety and efficacy of single-agent lenalidomide in subjects with relapsed/refractory mantle cell lymphoma: long-term follow-up analysis of the NHL-003 Study [abstract]. Blood 2012;120:Abstract 2738. This multicenter, Phase II study established the efficacy of lenalidomide as single-agent therapy in relapsed/refractory MCL and was the basis for the FDA approval for lenalidomide in this population of patients.
Habermann TM, Lossos IS, Justice G, Vose JM, Wiernik PH, McBride K, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle-cell lymphoma. Br J Haematol. 2009;145:344–9.
Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol. 2012;13:716–23.
Martin P, Jung S, Johnson J, et al. CALGB 50803(Alliance): a phase 2 trial of lenalidomide plus rituximab in patients with previously untreated follicular lymphoma [abstract]. Lugano June 2013.
Ruan J, Martin P, Shah B, et al. Combination biologic therapy without chemotherapy as initial treatment for mantle cell lymphoma: multi-center phase ii study of lenalidomide plus rituximab [abstract]. Blood 2013 abstract 247.
Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–74. This multicenter phase II study established the single-agent activity of bortezomib in relapsed/refractory MCL and was the basis for the FDA approval of the drug in relapsed/refractory MCL.
Goy A, Bernstein SH, Kahl BS, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analysis of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20:520–5.
Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92.
Advani RH, Buggy JJ, Sharman J, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. This was the phase I study of ibrutinib in relapsed/refractory B-cell malignancies that established toxicity, tolerability, and led to further phase II studies in MCL, DLBCL, FL, CLL, and Waldenstrom’s macroglobulinemia.
Wilson W, Gerecitano J, Goy A, et al. The Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib (PCI-32765), has preferential activity in the ABC subtype of relapsed/refractory de novo diff use large B-cell lymphoma (DLBCL): interim results of a multicenter, open-label, phase 2 study. Blood 2012;120(Suppl 1):Abstract 686. The interim results of this Phase II study in DLBCL established the efficacy of single-agent ibrutinib in ABC type DLBCL, which is dependent on chronic active BCR signaling while confirming its inability to produce similar results in the GCB type lymphoma. This will guide future studies of ibrutinib in DLBCL.
Fowler N, Advani R, Sharman J, et al. The Bruton’s tyrosine kinase inhibitor ibrutinib (PCI-32765) is active and tolerated in relapsed follicular lymphoma. Blood 2012;120(Suppl 1):Abstract 156.
Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–16. This important Phase II study established the efficacy of ibrutinib in relapsed/refractory MCL documenting significant responses and progression-free survival. The FDA accelerated approval of ibrutinib in MCL was based on the results of the patients treated on this trial.
Blum K. A phase I trial of the Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib (PCI-32765), in combination with rituximab (R) and bendamustine in patients with relapsed/refractory non-Hodgkin’s lymphoma (NHL) [abstract]. Blood 2012;Abstract 1643.
Younes A, Flinn I, Berdeja J, et al. Combining Ibrutinib with Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP): updated results from a Phase IB study in treatment-naïve patients with CD20 positive B-cell non-Hodgkin’s lymphoma (NHL) [abstract]. Blood 2013: Abstract 852.
Gopal A, Kahl BS, De Vos S, et al. Mature response data from a phase 2 study of PI3K-delta inhibitor idelalisib in patients with double (rituximab and alkylating agent)-refractory indolent B-cell non-Hodgkin lymphoma (iNHL) [abstract]. Blood 2013:Abstract 85.
Davids MS, Roberts AW, Anderson MA, et al. The BCL-2-specific BH3-mimetic ABT-199 (GDC- 0199) is active and well-tolerated in patients with relapsed non-Hodgkin lymphoma: interim results of a phase I study [abstract]. Blood. 2012;120:404.
Kahl B, Patel M, Younes A, et al. Preliminary safety and efficacy of IPI-145, a potent inhibitor of phosphoinositide-3-kinase-δ-γ in patients with relapsed/refractory B-cell lymphoma.
Friedberg JW, Cebula E, Burack R, et al. Phase II study of Alisertib, a selective aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. J Clin Oncol 2013 Sep 16 [Epub ahead of print].
Conflict of Interest
Kami Maddocks and Kristie A. Blum have both received research funding from Pharmacyclics.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maddocks, K., Blum, K.A. Ibrutinib in B-cell Lymphomas. Curr. Treat. Options in Oncol. 15, 226–237 (2014). https://doi.org/10.1007/s11864-014-0274-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-014-0274-8