Abstract
Processing of novel sintered steels with compositions including oxygen-sensitive elements requires deep understanding of the chemistry of sintering. The use of H2 atmospheres alleviates the oxygen transference from the base powder to the oxygen-sensitive particles. However, in H2, methane formation at 700–1200°C causes dramatic homogeneous decarburization of the part that affects both mechanical behavior and dimensional stability. The intensity and the critical temperatures of this effect depend strongly on the alloying elements, being significantly enhanced in presence of Si. When combining the alloying elements as Fe-Mn-Si masteralloys, methane formation is enhanced around 760°C due to the high Mn content (40 wt.%) in the masteralloys. Nevertheless, the benefits of H2 towards oxide reduction can still be advantageously used if diluting it in the form of N2-H2 atmospheres, or if limiting the use of H2 to temperatures below 500°C. Thus, decarburization due to methane formation can be successfully controlled.
Similar content being viewed by others
Introduction
The production of low alloy steels containing oxidation-sensitive elements such as Cr, Mn and Si offers the chance to obtain attractive properties at moderate and stable alloying cost. However, sintering this type of steels requires a profound knowledge of the chemical reactions occurring during the process.1,2,3,4,5,6,7 It is well known that, when such alloying elements are introduced by admixing—either as elemental particles or as masteralloys (MA)—a significant amount of oxygen is introduced through the base iron powder itself and then transferred to the alloying particles as a consequence of the so-called “internal getter effect”.8,9,10,11,12,13 One possible way to avoid oxygen transference from the iron base powder to the oxygen sensitive particles is to reduce the iron oxides with H2 during the early stages of the sintering cycle (~400°C) when the reactivity of the alloy elements is still low.2,13 However, in studies of sintering cycles carried out in H2, the formation of methane at intermediate temperatures (~700–900°C) has been observed, and it seems to be related to an enhanced homogeneous decarburization as compared to samples sintered in inert atmospheres.13,14
Two different decarburization processes can be distinguished: homogeneous or heterogeneous. Homogeneous decarburization normally occurs due to the consumption of carbon for the reduction of the oxides naturally present in all metallic powders. It is fairly reproducible and regularly distributed within the sample, as the initial oxygen distribution is also regular. Homogeneous decarburization is inevitable and has to be considered when defining the amount of graphite to be admixed in the sample. On the other hand, heterogeneous decarburization (or surface decarburization) may easily happen in cases of improper atmosphere control. This type of decarburizing reaction starts at the surface and proceeds into the core, the faster and deeper the higher the sintering temperature.
Homogeneous decarburization should be more intense under inert atmospheres as compared to H2 atmospheres. In the former ones, the reduction of oxides takes place entirely by reaction with carbon, and therefore the expected carbon loss is higher than in the case of H2 atmospheres in which a considerable fraction of the oxides is reduced by H2 at low temperatures.2,3 However, in steels containing oxygen-sensitive elements, homogeneous decarburization is significantly more severe in H2 atmospheres, which suggests that the methane formation process might entail a relevant risk for carbon control in this type of steels.13,14
This work presents a detailed study on the methane formation process observed in the heating section of the sintering cycle and evaluates its consequences for carbon control in the final sintered component. The effect of the atmosphere composition is assessed in order to find suitable conditions that provide both effective oxide reduction and reasonable carbon control, thus ensuring a robust mechanical performance.
Experimental Procedure
The chemical reactions during sintering were studied by thermal analysis techniques using a high-performance modular Simultaneous Thermal Analyzer Netzsch STA 449 C, coupled with a quadrupol mass spectrometer (Netzsch Aeolos) by a heated quartz capillary. Mass spectrometry (MS) allowed the analysis of the gaseous species evolved during the sintering cycle. In these experiments, the masses registered were 12(C), 14(N), 15(CH3), 16 (CH4, O), 17(OH), 18(H2O), 28(CO, N2), 32(O2) and 44(CO2).
Thermal analysis were carried out on different combinations of loose powder mixes (not pressed) prepared using Fe base powder (ASC100.29), graphite (grade UF4, Kropfmühl) and different additives: elemental Si, Mn and Cr powder, or three different master alloys (MA1: Fe-40Mn-17Si, MA2:Fe-40Mn-15Si-1C and MA3: Fe-40Mn-10Si-15Cr-0.5C, compositions in wt.%). See details in Supplementary Tables I and II. Standard tensile test bars (ISO 2740) were produced by pressing Fe-0.5%C-4%MA mixes at 600 MPa in a double-action press. Sintering was carried out in a laboratory-scale furnace AHT Silitstabofen at 1250°C for 30 min under different atmospheres: Cycle 1 in N2, Cycle 2 in H2, Cycle 3 in N2-5H2 and Cycle 4 in H2 up to 500°C and then in N2. The heating rate was ~0.167°C/s and the cooling rate from 900°C was approximately 1°C/s. See details in Supplementary Table III. Tensile tests were performed following the standard procedure defined in UNE-EN 10002-1:2002.15 The level of oxygen and carbon in the sintered samples was analyzed using LECO-TC400 and LECO-CS230, respectively.
Thermal Analysis in Steels Containing Oxygen Sensitive Masteralloys
Figure 1 shows the thermogravimetry and degassing curves obtained from Fe-0.5C steels that contain small additions (4 wt.%) of the MAs studied, for sintering runs in inert (Ar: Fig. 1, left) and reducing (H2: Fig. 1, right) atmospheres. The internal getter effect (described in detail in Refs. 8, 10, and 13) is clearly observed in MA-containing steels processed in Ar atmospheres. The typical degassing peak at ~700–800°C that indicates the reduction of Fe oxides in unalloyed Fe-0.5C steels is considerably less intense in the presence of MA particles. This is due to the fact that the MA powder particles immediately react with the gaseous products of the reduction of Fe-oxides. As a consequence, the m28 (CO) peak typically observed in Fe powders is reduced in intensity, and the mass loss is considerably lower than in Fe-0.5C compacts (because the oxygen is simply transferred from the iron powder to the MAs). In the case of MA3—with a higher Cr content—this effect is even more intense: the m28 (CO) peak is almost completely absent and no mass losses are observed at this temperature.
The internal getter effect is alleviated by using H2 atmospheres (Fig. 1, right). At low temperatures, H2 is the most effective reducing agent and eliminates the surface iron oxides at ~400°C forming m18 (H2O).2,3 At this temperature, the oxygen-sensitive elements are still not so reactive with the atmosphere. At higher temperatures, the situation changes and the most effective reducing agent is always carbon,2,3 therefore the reduction processes are detected as peaks of m28 (CO). The graphs shown in Fig. 1, right indicate how the reduction of surface iron oxides is effectively taking place at ~400°C, even when MA powders are added. However, in this latter case, two peculiar effects are observed in steels containing MA: a slight mass gain at temperatures above 400°C and an enhanced formation of methane indicated by the m16 (CH4) peaks at ~760°C and ~900°C. The m16 observed in the mass spectrometer can indicate either the presence of methane (CH4) or that of oxygen (O). A reliable confirmation of the presence of CH4 is the m15 (CH3*) signal that closely follows the signal of m16.
Detailed Study of Methane Formation
The Gibbs free energy of the possible reactions leading to methane generation through the interaction between gases present in the atmosphere (Eqs. 1, 2, 3, and 4) is positive in most cases at temperatures above 600°C, which indicates a low probability of forming methane through these mechanisms at the temperatures at which methane is observed in this study.
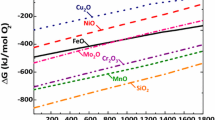
On the other hand, methane formation seems to be enhanced by the presence of MA powders that contain oxygen-sensitive alloying elements. Therefore, a possible mechanism could be one involving the oxidation of oxygen-sensitive elements, such as the one shown in Eq. 5, which—for the formation of Cr, Mn and Si oxides—presents a negative Gibbs free energy (see Supplementary Fig. 1) in the range of temperatures where methane is observed (700°C and 1200°C).
Even though the presence of oxygen-sensitive alloying elements seems to promote the formation of methane, thermal analyses in H2 for mixes containing graphite and Si, Mn, Cr powders (without the presence of Fe) do not show any methane (see Fig. 2). However, when elemental particles of Cr, Mn and Si are admixed in small amounts with a base iron powder (4 wt.%), methane is detected, and the peak is particularly intense in mixes with Si (see Fig. 3) This suggests that the interaction with the iron base powder plays an important role, probably by providing CO from the reduction of iron oxides present in the base powder. The onset of the methane peak (m16) observed in experiments with mixes (Figs. 1, right and 3) seems to be linked with the onset of the CO peak (m28). This could be indicating that the presence of CO is needed to form methane within the critical temperature range in which the reaction is viable. Also, in the reaction proposed in Eq. 5, CO is needed to form CH4.
The situation is different when the oxygen-sensitive alloying elements are combined in the form of a highly alloyed MA powder that contains maximum 50 wt.% Fe. Analysis of the surface of the MA particles used in this study (by x-ray photoelectron spectroscopy)16 showed that most of the powder surface is covered by an easily reducible iron oxide layer with a thickness of approximately 1 nm. Thus, reduction of such iron oxide could form the CO that seems to be needed for the formation of CH4, even without admixed iron base powder.
Thermal analysis in powder mixes containing graphite and MA (without the presence of Fe) are presented in Fig. 4. The experiments in Ar (Fig. 4, left) show a first CO peak linked with a mass loss at approximately 1070–1100°C, indicating the beginning of the reduction process. In inert atmospheres, carbothermal reduction of iron oxides would be expected to occur at temperatures around 700°C. However, as is well known from previous studies on Cr-prealloyed powders,2,3 when elements with high oxygen affinity are introduced by prealloying, an internal getter effect takes place in this case, not through the atmosphere but within the powder particle itself.8 As a consequence, the iron surface oxides are transformed into more stable oxides, and the reduction processes are shifted to higher temperatures. In Cr-prealloyed powders, the reduction is typically shifted to temperatures around 1100°C.2,3 Also, in the case of the MA powders used in this study, the reduction reactions are shifted to similar temperature ranges. When the experiments with MA1 +0.5C and MA2 +0.5C mixes are carried out in H2 atmosphere (see Fig. 4, right), the CO peak observed at approximately 1070–1100°C is closely followed by an intense peak of CH4 (m16), which again suggests that the formation of CH4 is linked to that of CO. However, in the case of mixes with MA3 (with 15 wt.%Cr but less Si in its composition), the formation of methane starts at slightly lower temperatures and is less intense than with MA1 and MA2.
Besides the methane (m16) peak observed at high temperatures (900–1060°C), another methane peak is formed in MA + C mixes at approximately 400°C. It is exactly at this temperature that the reduction of iron oxides with H2 (forming H2O as a product) would be expected to occur. However, neither a mass loss nor a H2O (m18) peak are observed at this temperature. This might be again indicating some type of internal getter effect, in this case H2O being the “transferring agent”. In any case, it has to be considered that at such low temperatures the reactions from Eqs. 1, 2, 3, and 4 would also be viable mechanisms.
A detail on the methane peaks observed during this study is presented in Fig. 5. In this case, only the m15 signal was represented, as it is a more accurate indication of the formation of CH4 than the m16 signal itself.
Some methane formation is observed in unalloyed Fe-0.5C mixes, which could be due to the presence of Mn and Si traces (impurities) in the starting water atomized iron powder. When small amounts of MA powder (4 wt.%) are added to the mix (Fe-0.5C-4MA), formation of methane occurs at considerably lower temperatures than in unalloyed Fe-0.5C (see Fig. 5a). The process is particularly enhanced at 770°C where an intense CH4 peak is found for all MA compositions, and a second peak is observed at approximately 910°C. The enhancement of methane formation in Fe-0.5C-4MA mixes occurs at lower temperatures (~770°C) than those at which methane is observed in MA + 0.5C mixes (~1050°C for MA1 and MA2, see Fig. 5b). In addition, in this latter case, the shape of the methane peak seems to depend on the MA composition, while in Fe-0.5C-4MA mixes, the shape of the CH4 peak is very similar independently on the MA compositions.
A detail on the methane peaks in mixes with elemental alloying particles, Fe-0.5C-4(Si, Mn, Cr), is presented in Fig. 5c. In this case, addition of Cr does not significantly affect the methane formation, presenting a peak that is very similar to that of Fe-0.5C mixes. The intensity of the methane peak increases with addition of Mn, and the process is shifted to lower temperatures. In mixes with Mn, two main peaks are found in the CH4 signal: at ~780°C and at ~965°C, but the most intense methane peaks are observed in mixes with Si. In this case, the formation of CH4 is shifted to high temperatures, and at those temperatures that are critical for Mn (700–960°C), the methane formation in mixes with Si powders is negligible. This is in agreement with previous studies on the internal getter effect carried out in mixes with elemental powder particles, which indicated that Si powders have a very low reactivity up to temperatures around 900°C10 due to the presence of a protective oxide on the surface of the Si particles. Methane formation in mixes with Si occurs abruptly at ~1000–1060°C with a very intense CH4 peak. Two phenomena seem to be linked to the formation of CH4 at this specific temperature (see Fig. 6): the sudden increase in the intensity of the CO peak, and an intense exothermic process observed in the DTA signal. The exothermic process is most likely linked to the formation of an Fe3Si intermetallic compound in the areas of contact between Fe and Si powder particles.10,17 The formation of an intermetallic compound from an Fe-0.5C metallic matrix leads to a carbon depletion in these areas and thus can facilitate the elimination of carbon from the sample forming CH4 or CO.
A global view of these results shows that methane formation becomes more intense as the oxygen affinity of the elements increases (Si > Mn > Cr). In addition, the tendency to form methane is different for different alloying elements, Mn and Cr being more sensitive at around 700–1000°C and Si at 1000–1100°C. Such temperature ranges are in agreement with the temperature ranges of highest sensitivity to the internal getter effect for these elemental alloying powders.10
Effects of the Atmosphere on the Sintered Components
The study of methane formation is important because it can have dramatic consequences for the properties of the final sintered component. In order to study this effect, four different atmospheres have been used for sintering: plain N2 (Cycle 1), plain H2 (Cycle 2), diluted N2-5 vol%H2 (Cycle 3) and a mixed cycle with H2 up to 500°C that is then switched to N2 (Cycle 4). The aim of using Cycle 4 is to introduce H2 in just that temperature range where it is needed for reduction.
Metallographic images of samples sintered in these four atmospheres (Fig. 7) clearly show the homogeneous decarburization that takes place when sintering in H2, as compared to the treatment in N2. This decarburization can be effectively controlled by either diluting H2 in N2 (Cycle 3-N2-5H2), or by using H2 only up to 500°C (Cycle 4). In these two last cases H2 is used to advantage for reducing the oxides (oxygen contents are lower than in Cycle 1-N2), and at the same time decarburization due to methane formation is avoided (see oxygen and carbon contents in Fig. 7; Supplementary Table IV and Supplementary Fig. 2).
Decarburization has a pronounced effect on mechanical properties (see Fig. 8). Due to the significant carbon losses, the samples sintered in H2 have lower ultimate tensile strength and consequently higher elongation values. Also, the dimensional stability of the sintered parts is strongly affected by the sintering atmospheres, with a clear tendency towards higher shrinkage when using plain H2 atmospheres, and intermediate degrees of shrinkage for cycles in which the amount of H2 is controlled (Cycles 3 and 4). A similar tendency is observed in sintered density, which is slightly higher in H2 (7.05 ± 0.02 g/cm3 for MA2) than in plain N2 atmospheres (7.00 ± 0.02 g/cm3 for MA2), and presents intermediate values when the amount of H2 is controlled (~7.03 ± 0.02 g/cm3 for MA2 when using Cycles 3 and 4).
Conclusion
A detailed study on the methane formation observed in steels containing oxidation-sensitive elements has shown that the intensity and the critical temperatures of the process strongly depend both on the alloying elements used and on their interaction with the Fe base powder. Small additions of Cr and Mn to an Fe-0.5C steel produce methane at temperatures around 700–900°C, while additions of Si shift the process to higher temperatures (~1060°C) and increase its intensity significantly. When these elements are combined in the form of an Fe-Mn-Si-(C-Cr) masteralloy powder, methane formation is particularly enhanced at ~760°C for all the MA compositions used, which suggests that the process is dominated by the high Mn content in the MA powders used.
As a consequence of the methane formation, steels containing MA additions and sintered in plain H2 show pronounced homogeneous decarburization, resulting in a dramatic decrease in the ultimate tensile strength and a consequent increase in elongation. The dimensional changes of the sintered components are also strongly affected. Significantly higher shrinkage is observed in plain H2 as compared to N2. In practice, this means that the conditions of the atmosphere, and thus the chemical reactions occurring during sintering, will affect both the mechanical performance and the dimensional stability of the sintered parts.
The extreme consequences of methane formation observed in pure H2 atmospheres can be avoided in two ways: by the use of diluted N2-H2 atmospheres or by limiting the use of H2 to temperatures below 500°C and then shifting to N2 at higher temperatures to avoid the methane formation reaction. In these last two cases, homogeneous decarburization is controlled, effective reduction of oxides is achieved, and a slight increase in strength values is observed as compared to plain N2 atmospheres.
References
D. Chasoglou, E. Hryha, and L. Nyborg, Mater. Chem. Phys. 138, 405 (2013).
H. Danninger and C. Gierl, Mater. Chem. Phys. 67, 49 (2001).
H. Danninger and C. Gierl, Sci. Sinter. 40, 33 (2008).
H. Danninger, C. Xu, and B. Lindqvist, Prog. Powder Metall 534–536, 577 (2007).
E. Hryha, S. Karamchedu, D. Riabov, L. Nyborg, and S. Berg, J. Am. Ceram. Soc. 98, 3561 (2015).
E. Hryha and L. Nyborg, Metall. Mater. Trans. A 45, 1736 (2014).
S. Karamchedu, E. Hryha, and L. Nyborg, J. Mater. Process. Technol. 223, 171 (2015).
C. Gierl-Mayer and R. Oro, Calderon, and H. Danninger, JOM 68, 920 (2016).
R. Oro, C. Gierl-Mayer, and H. Danninger, Powder Metall. (2016). doi:10.1080/00325899.2016.1269430.
R. Oro, C. Gierl, and H. Danninger, J. Therm. Anal. Calorim. 127, 91 (2017).
E. Hryha and E. Dudrova, Prog. Powder Metall 534–536, 761 (2007).
E. Hryha, E. Dudrova, and L. Nyborg, Metall. Mater. Trans. A 41A, 2880 (2010).
R. Oro, M. Campos, C. Gierl-Mayer, H. Danninger, and J. Torralba, Metall. Mater. Trans. A 46, 1349 (2015).
Danninger, H., A. Avakemian, C. Gierl, M. Dlapka, and M. Grafinger. in Proc. European International Powder Metallurgy Congress and Exhibition EuroPM 2014 (Salzburg, Austria, 2014).
ISO 6892-1:2009, in Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. International Organization for Standardization (Geneva, Switzerland).
Oro, R., M. Campos, E. Hryha, L. Nyborg, and J.M. Torralba. in Proc. European International Powder Metallurgy Congress and Exhibition Euro PM 2011 (Barcelona, Spain, 2011).
Y. Zhang and D. Ivey, J. Mater. Sci. 33, 3131 (1998).
Acknowledgements
Open access funding provided by TU Wien (TUW). The research leading to these results has received funding from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme FP7/2007–2013/under REA Grant Agreement No. 625556. The support from the European Research Commission through the People Work Program FP7-PEOPLE-2013-IEF is very gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
de Oro Calderon, R., Jaliliziyaeian, M., Gierl-Mayer, C. et al. Effects of H2 Atmospheres on Sintering of Low Alloy Steels Containing Oxygen-Sensitive Masteralloys. JOM 69, 635–644 (2017). https://doi.org/10.1007/s11837-017-2287-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-017-2287-9