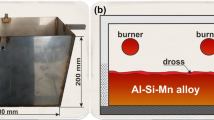
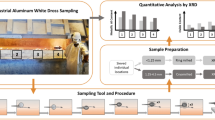
Abstract
The salt removal from black dross by thermal treatment has experimentally been studied under different conditions in both a stationary resistance furnace and in a laboratory scale rotary furnace. The experiments were designed based on partial pressure calculations using the Thermo-Calc software (Thermo-Calc Software, Stockholm, Sweden). The salt removal efficiency was evaluated by scanning electron microscope (SEM) energy-dispersive x-ray spectroscopy and x-ray diffraction analyses, and the optimum conditions for treatment established, i.e., temperature, gas flow rate, holding time, rotation rate, and sample size. The overall degree of chloride removal was established to increase as a function of time and temperature, as well as by reduced pressure. Under atmospheric pressure, the highest degree of chloride removal from a 20 g sample was obtained after 10 h at 1523 K resulting in a 98% removal and a final chloride content of 0.3 wt.% in the residue. Under reduced pressure, the chloride concentrate was lowered to 0.2 wt.% after thermal treatment of a 20 g sample at 1473 K for 8 h. In the case of 200 g samples treated in a rotary furnace, the chloride concentrate was 2.5 wt.% after 14 h at 1523 K, representing a removal of 87%. Below 0.3 wt.% chloride content, the material is deemed a nonhazardous waste.













Similar content being viewed by others
References
A. Gil, Ind. Eng. Chem. Res. 44, 8852 (2005).
J.A. Tenorio and D.C.R. Espinosa, Handbook of Aluminum: Alloy Production and Manufacturing, ed. G.E. Totten and M.D. Scott (Boca Raton, FL: CRC Press, 2003), pp. 115–155.
M.E. Schilesinge, Aluminium Recycle, 2nd ed. (Boca Raton, FL: Taylor & Francis, 2013).
O. Majidi, S.G. Shabestari, and M.R. Aboutalebi, J. Mater. Process. Technol. 182, 450 (2007).
C.F. Beaur, J.H. Hussey, B. Langston, and N.M. Parikh, U.S. patent 4501614, 1985.
X.-L. Huang, A.E. Badawy, M. Arambewela, R. Ford, M. Barlaz, and T. Tolaymat, J. Hazard. Mater. 273, 192 (2014).
P.E. Tsakiridis, P. Oustadakis, and S. Agatzini-Leonardou, J. Environ. Chem. Eng. 1, 23 (2013).
W.J. Bruckard and J.T. Woodcock, Miner. Eng. 20, 1376 (2007).
S.R. Rao, By-Product Processing and Utilization (New York: Elsevier, 2006).
H. Shen and E. Forssberg, Waste Manag 23, 942 (2003).
O. Manfredi, W. Wuth, and I. Bohlinger, JOM 49(11), 48 (1997).
M. Davies, P. Smith, W.J. Bruckard, and J.T. Woodcock, Miner. Eng. 21, 605 (2008).
J.N. Hryn and E.J. Daniels, Recycling of Aluminium Salt Cake (2001), http://www.idalsa.com/wp-content/themes/idalsa/pdf/IDALSA,%20RECYCLING%20ALUMINIUM%20SALT%20CAKE.pdf.
J. Gerber, International Aluminum Institute, London, UK, 2009.
M.C. Shinzato and R. Hypolito, Waste Manag. 25, 37 (2005).
Y. Xiao, M.A. Reuter, and U.D.O. Boin, J. Environ. Sci. Health. Part A 40, 1861 (2005).
D. Yerushalmi, U.S. patent 5102453, 1992.
B.J. Jody and E.J. Daniels (Paper presented at the Seventh International Conference on Solid Waste Management and Secondary Materials, December 1991).
J.N. Hryn (Paper presented at the Global Symposium on Recycling, Waste Treatment and Clean Technology, Cancun, Mexico, 2008).
S.A. Korili and A. Gil, Hazwaste Manag. A5.3, 1 (2008).
C. Schmitz, Handbook of Aluminium Recycling: Fundamentals, Mechanical Preparation, Metallurgical Processing, Plant Design, ed. Ch. Schmitz (Essen, Germany: Vulkan-Verlag GmbH, 2006), pp. 150–157.
T. Tzonev and B. Lucheva, JOM 59(11), 64 (2007).
A. Gil, Environ. Eng. Sci. 24, 1234 (2007).
Acknowledgements
The authors would like to thank the Department of Materials Science and Engineering at the Norwegian University of Science and Technology (NTNU) in Trondheim, Norway, for the use of the rotary furnace, as well as Harold Holm for helping with construction of the experiment apparatus. The supply of black dross from Stena Aluminum AB, Älmhult, Sweden, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beheshti, R., Moosberg-Bustnes, J., Akhtar, S. et al. Black Dross: Processing Salt Removal from Black Dross by Thermal Treatment. JOM 66, 2243–2252 (2014). https://doi.org/10.1007/s11837-014-1178-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-014-1178-6