Abstract
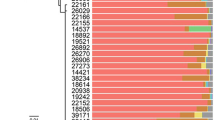
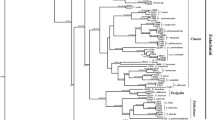
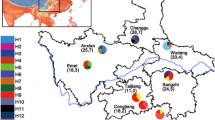
Microbial symbionts have come to be recognized as agents in the speciation of their eukaryote hosts. In this study, we asked if bacterial symbionts are, or were in the past, involved in the speciation of the gall-inducing aphid Slavum wertheimae (Hemiptera: Aphididae). This aphid is specific to the tree Pistacia atlantica, which has a fragmented distribution among mesic and xeric habitats, leading to corresponding fragmentation of the aphid population. Previous studies revealed genetic differentiation among populations of the gall-inducing aphid, suggesting cryptic allopatric speciation. Pistacia atlantica trees show no such variation. By means of diagnostic PCR, we screened several populations of S. wertheimae from mesic and xeric sites in Israel for the presence of nine known aphid symbionts: Arsenophonus, Hamiltonella, Regiella, Rickettsia, Rickettsiella, Serratia, Spiroplasma, Wolbachia, and X-type, as well as Cardinium, known to be a reproductive manipulator. Only one symbiont, Wolbachia, was detected in S. wertheimae. Wolbachia was found in all the aphids of the mesic populations, compared to 26% in the aphids from the xeric populations. Multilocus Sequence typing of Wolbachia revealed new haplotypes in the fbpA and coxA genes in both the mesic and xeric populations. Phylogenetic analysis showed that Wolbachia of S. wertheimae is closely related to Wolbachia strains from assorted hosts, mostly lepidopterans, but only distantly related to Wolbachia strains from other aphid species. We conclude that the cryptic speciation of mesic and xeric populations of S. wertheimae was likely driven by geographical isolation rather than by Wolbachia.



Similar content being viewed by others
References
Augustinos AA, Santos-Garcia D, Dionyssopoulou E et al (2011) Detection and characterization of Wolbachia infections in natural populations of aphids: Is the hidden diversity fully unraveled? PLoS One 6:e28695
Avrani S, Ben-Shlomo R, Inbar M (2012) Genetic structure of a galling aphid Slavum wertheimae and its host tree Pistacia atlantica across an Irano-Turanian distribution: from fragmentation to speciation?. Tree Genet Genom 8: 811–820
Baldo L, Hotopp JCD, Jolley KA et al (2006) Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol 72:7098–7110
Bennett GM, Moran NA (2015) Heritable symbiosis: the advantages and perils of an evolutionary rabbit hole. Proc Natl Acad Sci 112: 10169–10176
Bolnick DI, Fitzpatrick BM (2007) Sympatric speciation: models and empirical evidence. Annu Rev Ecol Evol Syst 38: 459–487
Bordenstein SR, O’Hara FP, Werren JH (2001) Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 409:707–710
Brucker RM, Bordenstein SR (2012) Speciation by symbiosis. Trends Ecol Evol 27:443–451
Brucker RM, Bordenstein SR (2013) The hologenomic basis of speciation: gut bacteria cause hybrid lethality in the genus Nasonia. Science 341: 667–669
Butlin RK, Galindo J, Grahame JW (2008) Sympatric, parapatric or allopatric: the most important way to classify speciation?. Philos Trans R Soc Lond B Biol Sci 363: 2997–3007
Correa CC, Ballard JWO (2016) Wolbachia associations with insects: Winning or losing against a master manipulator. Front Ecol Evol 3:153
Danin A (1999) Sandstone outcrops: a major refugium of Mediterranean flora in the xeric part of Jordan. Isr J. Plant Sci 47:179–187
Douglas AE (2015) Multiorganismal insects: diversity and function of resident microorganisms. Annu Rev Entomol 60:17–34
Engelstädter J, Hurst GDD (2009) The ecology and evolution of microbes that manipulate host reproduction. Annu Rev Ecol Evol Syst 40: 127–149
Ferrari J, West JA, Via S, Godfray HCJ (2012) Population genetic structure and secondary symbionts in host-associated populations of the pea aphid complex. Evolution 66: 375–390
Gilbert SF, Bosch TCG, Ledón-Rettig C (2015) Eco-Evo-Devo: developmental symbiosis and developmental plasticity as evolutionary agents. Nat Rev Genet 16:611–622
Heddi A, Grenier A-M, Khatchadourian C et al (1999) Four intracellular genomes direct weevil biology: nuclear, mitochondrial, principal endosymbiont, and Wolbachia. Proc Natl Acad Sci 96: 6814–6819
Inbar M, Kark S (2007) Gender-related developmental instability and herbivory of Pistacia atlantica across a steep environmental gradient. Folia Geobot 42: 401–410
Jeyaprakash A, Hoy MA (2000) Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol Biol 9:393–405
Jones RT, Bressan A, Greenwell AM, Fierer N (2011) Bacterial communities of two parthenogenetic aphid species cocolonizing two host plants across the Hawaiian Islands. Appl Environ Microbiol 77:8345–8349
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Li YY, Floate KD, Fields PG, Pang BP (2014) Review of treatment methods to remove Wolbachia bacteria from arthropods. Symbiosis 62:1–15
McLean AHC, Parker BJ, Hrček J et al (2016) Insect symbionts in food webs. Philos Trans R Soc Lond B Biol Sci 371:20150325
Moran NA, Russell JA, Koga R, Fukatsu T (2005) Evolutionary relationships of three new species of enterobacteriaceae living as symbionts of aphids and other insects. Appl Environ Microbiol 71:3302–3310
Moran NA, McCutcheon JP, Nakabachi A (2008) Genomics and evolution of heritable bacterial symbionts. Ann Rev Genet 42:165–190
Nakamura Y, Kawai S, Yukuhiro F et al (2009) Prevalence of Cardinium bacteria in planthoppers and spider mites and taxonomic revision of “Candidatus Cardinium hertigii” based on detection of a new Cardinium group from biting midges. Appl Environ Microbiol 75:6757–6763
Oliver KM, Martinez AJ (2014) How resident microbes modulate ecologically-important traits of insects. Curr Opin Insect Sci 4: 1–7
Russell JA, Weldon S, Smith AH et al (2013) Uncovering symbiont-driven genetic diversity across North American pea aphids. Mol Ecol 22:2045–2059
Schluter D (2009) Evidence for ecological speciation and its alternative. Science 323:737–741
Simon J-C, Boutin S, Tsuchida T et al (2011) Facultative symbiont infections affect aphid reproduction. PLoS One 6:e21831
Skaljac (2016) Bacterial Symbionts of Aphids (Hemiptera: Aphididae). In: Vilcinskas E (ed) Biology and Ecology of Aphids, CRC press, Boca Raton, pp 100–125
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Taylor GP, Coghlin PC, Floate KD, Perlman SJ (2011) The host range of the male-killing symbiont Arsenophonus nasoniae in filth fly parasitioids. J Invertebr Pathol 106:371–379
Tsuchida T, Koga R, Shibao H et al (2002) Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol Ecol 11:2123–2135
Vavre F, Kremer N (2014) Microbial impacts on insect evolutionary diversification: from patterns to mechanisms. Curr Opin Insect Sci 4: 29–34
Vogel KJ, Moran NA (2013) Functional and evolutionary analysis of the genome of an obligate fungal symbiont. Genom Biol Evol 5:891–904
Wang Z, Shen Z-R, Song Y et al (2009) Distribution and diversity of Wolbachia in different populations of the wheat aphid Sitobion miscanthi (Hemiptera: Aphididae) in China. Eur J Entomol 106: 49–55
Wang Z, Su X-M, Wen J et al (2014) Widespread infection and diverse infection patterns of Wolbachia in Chinese aphids. Insect Sci 21: 313–325
Weinert LA, Araujo-Jnr E V., Ahmed MZ, Welch JJ (2015) The incidence of bacterial endosymbionts in terrestrial arthropods. Proc R Soc B 282: 20150249
Wool D (2004) Galling aphids: specialization, biological complexity, and variation. Annu Rev Entomol 49:175–192
Wool D, Bogen R (1999) Ecology of the gall-forming aphid, Slavum wertheimae, on Pistacia atlantica: population dynamics and differential herbivory. Isr J Zool 45:247–260
Zchori-Fein E, Bourtzis K (2012) Manipulative tenants: bacteria associated with arthropods. CRC Press, Boca Raton
Zug R, Hammerstein P (2015) Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol Rev 90:89–111
Zytynska SE, Weisser WW (2016) The natural occurrence of secondary bacterial symbionts in aphids. Ecol Entomol 41: 13–26
Acknowledgements
We would like to thank Moshe Inbar, Einat Zchori-Fein, and three anonymous reviewers for critical comments on earlier versions of the manuscript. We also thank Kerry Oliver for supplying us specimens for positive controls. The study was funded by an internal research grant from “Oranim” College of Education, and in part by the Israel Science Foundation (Grant No. 276/14).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: John F. Tooker.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amit, L., Ben-Shlomo, R. & Chiel, E. Are microbial symbionts involved in the speciation of the gall-inducing aphid, Slavum wertheimae?. Arthropod-Plant Interactions 11, 475–484 (2017). https://doi.org/10.1007/s11829-017-9495-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-017-9495-7