Abstract
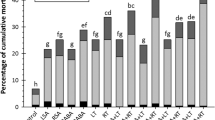
In Brazil, the silverleaf whitefly (SLW), Bemisia tabaci biotype B, is a serious soybean pest. SLW management is difficult and new control strategies, such as host–plant resistance, are required. Herein, we aimed to evaluate the biology of SLW, from eggs to adults, on seven soybean cultivars. The emergence of adult insects was monitored daily. Defense-related compounds were identified and quantified from the V3 to the V8 stages in SLW-infested and non-infested plants. The rates of emergence of SLW adults were lower in cultivars ‘IAC 17,’ ‘IAC 19’, and ‘IAC 24’ compared with the susceptible cultivar ‘IAC Holambra Stwart’. Rutin, genistin, genistein, and salicylic acid were identified and quantified in plant extracts. The rutin, genistin, and genistein levels decreased after SLW infestation. Rutin concentrations increased in infested plants of ‘IAC 17’ (V6) and ‘IAC 19’ (from V5 to V8). ‘Barreiras’ showed the highest genistein content in non-infested plants; from V5 growth stage, it was not detected in cultivars Doko (infested), Vencedora, ‘IAC 17’, and ‘IAC 24’ (non-infested). High levels of salicylic acid were observed in ‘IAC ‘19’-infested plants (at V3 and V5). The results suggest that rutin can be related to SLW adults’ emergence only when the feeding source was ‘IAC 19’. Consequently, further studies are needed to access the associated gene expressions and the effect of other secondary metabolites, mainly volatile compounds from SA pathway, including its consequences on feeding preference and mostly in relation to IAC cultivars.

Similar content being viewed by others
References
Baldin ELL, Fanela TLM, da Rocha Pannuti LE, Kato MJ, Takeara R, Crotti AEM (2015) Botanical extracts: additional tool for whitefly management in tomato. Hort Bras 33:59–65
Ballina-Gomez H, Ruiz-Sanchez E, Chan-Cupul W, Latournerie-Moreno L, Hernandez-Alvarado L, Islas-Flores I, Zuñiga-Aguilar JJ (2013) Response of Bemisia tabaci Genn. (Hemiptera: Aleyrodidae) biotype B to genotypes of pepper Capsicum annuum (Solanales: Solanaceae). Neotrop Entomo 42:205–210
Burr IW, Foster LA (1972) A test for equality of variances. University of Purdue, West Lafayette, p 26
Butter NS, Vir BK (1989) Morphological basis of resistance in cotton to the whitefly Bemisia tabaci. Phytoparasitica 17:251–261
Caarls L, Pieterse CM, Van Wees SC (2015) How salicylic acid takes transcriptional control over jasmonic acid signaling. Front Plant Sci 6:170
Carmona D, Lajeunesse MJ, Johnson MT (2011) Plant traits that predict resistance to herbivores. Funct Ecol 25:358–367
Coffey JL, Simmons AM, Shepard BM, Tadmor Y, Levi A (2015) Potential sources of whitefly (Hemiptera: Aleyrodidae) resistance in desert watermelon (Citrullus colocynthis) germplasm. HortScience 50:13–17
Cruz PL, Baldin EL, Maria de Jesus P (2014) Characterization of antibiosis to the silverleaf whitefly Bemisia tabaci biotype B (Hemiptera: Aleyrodidae) in cowpea entries. J Pest Sci 87:639–645
da Silva JPGF, Baldin ELL, de Souza ES, Lourenção AL (2012) Assessing Bemisia tabaci (genn.) biotype B resistance in soybean genotypes: antixenosis and antibiosis. Chil J Agric Res 72:516–522
De Barro PJ, Liu S-S, Boykin LM, Dinsdale AB (2011) Bemisia tabaci: a statement of species status. Ann Rev Entomol 56:1–19
Elzinga DA, De Vos M, Jander G (2014) Suppression of plant defenses by a Myzus persicae (green peach aphid) salivary effector protein. Mole Plant Microbe Interact 27:747–756
Fehr WR, Caviness CE (1977) Stages of soybean development. Iowa State University of Science and Technology, Ames
Fontes FHM, Colombo CA, Lourenção AL (2012) Structure of genetic diversity of Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae) populations in Brazilian crops and locations. Sci Agric 69:47–53
Freeman TP, Buckner JS, Nelson DR, Chu CC, Henneberry TJ (2001) Stylet penetration by Bemisia argentifolii (Homoptera: Aleyrodidae) into host leaf tissue. Ann Entomol Soc Am 94:761–768
Fugi CGQ, Lourenção AL, Parra JRP (2005) Biology of Anticarsia gemmatalis on soybean genotypes with different degrees of resistance to insects. Sci Agric 62:31–35
Gosset V, Harmel N, Göbel C, Francis F, Haubruge E, Wathelet JP, Fauconnier ML (2009) Attacks by a piercing-sucking insect (Myzus persicae Sultzer) or a chewing insect (Leptinotarsa decemlineata Say) on potato plants (Solanum tuberosum L.) induce differential changes in volatile compound release and oxylipin synthesis. J Exp Bot 15:1–10
Harborne JB (1967) Comparative biochemistry of the flavonoids. Academic Press, London
Harborne JB (1993) The flavonoids advances in reseach since 1986. Chapman & Hall, London
Hoffmann-Campo C (1995) Role of the flavonoids in the natural resistance of soybean to Helioths virescens (F) and Trichoplusia ni (Hübner). Doctoral dissertation, The University of Reading, Reading, UK
Hoffmann-Campo CB, Moscardi F, Corrêa-Ferreira BS, Oliveira LJ, Sosa-Gómez DR, Panizzi AR, Corso IC, Gazzoni DL, Oliveira Ed (2000) Pragas da soja no Brasil e seu manejo integrado [Integrated soybean pest management in Brazil], (Circular Técnica/Embrapa Soja, ISSN 1516-7860, no. 30 [Technical Newsletter]/Brazilian Agricultural Research Corporation—Soybean, International Standard Serial Number (ISSN) 1516-7860, no. 30)
Hoffmann-Campo CB, Harborne JB, McCafferry AR (2001) Pre-ingestive and post-ingestive effects of soybean extracts and rutin on Trichoplusia ni growth. Entomol Exp Appl 98:181–194
Hoffmann-Campo CB, Ramos Neto JA, Oliveira MCN, De Oliveira LJ (2006) Detrimental effect of rutin on a main soybean defoliator pest, Anticarsia gemmatalis. Pesq Agropecu Bras 41:1453–1459
Inbar M, Gerling D (2008) Plant-mediated interactions between whiteflies, herbivores, and natural enemies. Annu Rev Entomol 53:431–448
Karatolos N, Williamson MS, Denholm I, Gorman K, Ffrench-Constant RH, Bass C (2012) Over-expression of a cytochrome P450 is associated with resistance to pyriproxyfen in the greenhouse whitefly Trialeurodes vaporariorum. PLoS ONE 7(2):e31077
Kessler A, Baldwin IT (2004) Herbivore-induced plant vaccination. Part I. The orchestration of plant defenses in nature and their fitness consequences in the wild tobacco Nicotiana attenuata. Plant J 38:639–649. doi:10.1111/j.1365-313X.2004.02076.x
Koornneef A, Pieterse CMJ (2008) Cross talk in defense signaling. Plant Physiol 146:839–844
Koornneef A, Leon-Reyes A, Ritsema T, Verhage A, Den Otter FC, Van Loon LC, Pieterse CMJ (2008) Kinetics of salicylate-mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiol 147:1358–1368
Leitner M, Boland W, Mithöfer A (2005) Direct and indirect defences induced by piercing-sucking and chewing herbivores in Medicago truncatula. New Phytol 167:597–606
Leon-Reyes A, Du Y, Koornneef A, Proietti S, Körbes AP, Memelink J, Pieterse CM, Ritsema T (2010) Ethylene signaling renders the jasmonate response of Arabidopsis insensitive to future suppression by salicylic acid. Mol Plant Microbe Interact 23:187–197
Li Q, Tan W, Xue M, Zhao H, Wang C (2013) Dynamic changes in photosynthesis and chlorophyll fluorescence in Nicotiana tabacum infested by Bemisia tabaci (Middle East–Asia Minor 1) nymphs. Arthropod Plant Interact 7:431–443
Lo Presti L, Lanver D, Schweizer G, Tanaka S, Liang L, Tollot M, Kahmann R (2015) Fungal effectors and plant susceptibility. Annu Rev Plant Biol 66:513–545
Lourenção AL, Nagai H (1994) Surtos populacionais de Bemisia tabaci no Estado e São Paulo [Population outbreaks of Bemisia tabaci in the São Paulo state]. Bragantia 53:53–59
Lourenção AL, Pereira J, Miranda MD, Ambrosano GMB (2000) Avaliação de danos causados por percevejos e por lagartas em genótipos de soja de ciclos precoce e semiprecoce [Evaluation of damage to early maturity soybean cultivars and lines caused by stink bungs and caterpillars]. Pesq Agropecu Bras 35:879–886
Miranda MAC, Braga NR, Lourenção AL, Miranda FTS, Unêda SH, Ito MF (2003) Descrição, produtividade e estabilidade da cultivar de soja IAC-24, resistente a insetos [Description, yield and stability of soybean cultivar IAC-24 insect resistant.]. Bragantia 62:29–37
Mithöfer A, Boland W (2012) Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol 63:431–450
Moscardi F, Bueno AF, Sosa-Gómez DR, Roggia S, Hoffmann-Campo CB, Pomari AF, Corso IC, Yano SAC (2012) Artrópodes que atacam as folhas da soja. Soja: manejo integrado de insetos e outros artrópodes-praga [Arthropods which attack the soybean leaves. Soybeans: integrated management of insects and other arthropods pests.] Embrapa, Brasília, pp 213–334
Nauen R, Wölfel K, Lueke B, Myridakis A, Tsakireli D, Roditakis E, Vontas J (2015) Development of a lateral flow test to detect metabolic resistance in Bemisia tabaci mediated by CYP6CM1, a cytochrome P450 with broad spectrum catalytic efficiency. Pestic Biochem Physiol 121:3–11
Oliveira M, Henneberry T, Anderson P (2001) History, current status, and collaborative research projects for Bemisia tabaci. Crop Protect 20:709–723
Piubelli G (2004) Bioatividade de genótipos de soja resistentes a A. gemmatalis Hübner (Lepidoptera: Noctuidae) e interações de suas substâncias químicas com inimigos naturais [Bioactivity of soybean genotypes resistant to A. gemmatalis Hübner (Lepidoptera: Noctuidae) and its chemical interactions with natural enemies]. Doctoral Thesis, Federal University of Paraná, Curitiba, Paraná
Piubelli GC, Hoffmann-Campo CB, Moscardi F, Miyakubo SH, De Oliveira MCN (2005) Are chemical compounds important for soybean resistance to Anticarsia gemmatalis? J Chem Ecol 31:1509–1525
Rocha ABO, Lourenção AL, Miranda Filho HS, Hayashi PCR, Ramos VJ (2012) Resistência de clones de batata a Bemisia tabaci biótipo B [Resistance of potato clones to Bemisia tabaci biotype B]. Hortic Bras 30:32–38
Rosenthal GA, Berenbaum MR (2012) Herbivores: their interactions with secondary plant metabolites: ecological and evolutionary processes, vol 2. Academic Press, Cambridge
Salvador MC, Boiça AL Jr, De Oliveira MCN, Da Graça JP, Da Silva DM, Hoffmann-Campo CB (2010) Do different casein concentrations increase the adverse effect of rutin on the biology of Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae)? Neotrop Entomol 39:774–783
Sas Institute (2009) SAS/STAT: user’s guide: statistics, version 9.2. Statistical Analysis Systems, SAS Institute, Cary, NC
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611
Smith CM (2006) Plant resistance to arthropods. Molecular and conventional approach. Springer, Berlin
Srivastava R, Shukla Y, Kumar S (1999) Recent advances in the chemistry of insect antifeedants. J Med Aromat Plant Sci 21:59–76
Thaler JS, Humphrey PT, Whiteman NK (2012) Evolution of jasmonate and salycilate signal crosstalk. Trends Plant Sci 17:260–270
Tukey JW (1949) One degree of freedom for non-additivity. Biometrics 5:232–242
Valle GE, Lourenção AL (2002) Resistência de genótipos de soja a Bemisia tabaci (Genn.) biotype B (Hemiptera: Aleyrodidae) [Resistance of soybean genotypes to Bemisia tabaci (Genn.) biotype B (Hemiptera: Aleyrodidae)]. Neotrop Entomol 31:285–295
Valle GE, Lourenção AL, Pinheiro JB (2012) Adult attractiveness and oviposition preference of Bemisia tabaci biotype B in soybean genotypes with different trichome density. J Pest Sci 85:431–442
Valle GE, Lourenção AL, Zucchi MI, Pinheiro JB, De Abreu AG (2013) Population variability of Bemisia tabaci (Genn.) in different hosts. Genet Mol Res 12:4615–4624
VanDoorn A, de Vries M, Kant MR, Schuurink RC (2015) Whiteflies glycosylate salicylic acid and secrete the conjugate via their honeydew. J Chem Ecol 41:52–58
Vendramim J, Castiglioni E, Guedes J, Costa Id (2000) Aleloquímicos, resistência de plantas e plantas inseticidas [Allelochemicals, plant resistance and insecticide plants], in: Bases e técnicas do manejo de insetos. In: Guedes JC, Costa ID, Castiglioni E (eds) Santa Maria: Pallotti [Bases and methods of integrated management of insects], UFSM/CCR/DFS, Pallotti, Santa Maria, pp 113–28
Vieira SS, Bueno AF, Boff MIC, Bueno R, Hoffman-Campo CB (2011) Resistance of soybean genotypes to Bemisia tabaci (Genn.) biotype B (Hemiptera: Aleyrodidae). Neotrop Entomol 40:117–122
Vieira SS, Bueno RCOF, Bueno AF, Boff MIC, Gobbi AL (2013) Different timing of whitefly control and soybean yield. Cienc Rural 43:247–253
Zhang PJ, Zheng SJ, van Loon JJA, Boland W, David A, Mumm R, Dicke M (2009) Whiteflies interfere with indirect plant defense against spider mites in Lima bean. Proc Natl Acad Sci USA 106:21202–21207
Zhang P-J, Li W-D, Huang F, Zhang J-Z, Xu FC, Lu Y-B (2013) Feeding by whiteflies suppresses downstream jasmonic acid signaling by eliciting salicylic acid signaling. J Chem Ecol 39:612–619
Zhu-Salzman K, Zeng R (2015) Insect response to plant defensive protease inhibitors. Ann Rev Entomol 60:233–252
Acknowledgments
We are grateful to the São Paulo Research Foundation (Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP) for the Ph.D. scholarship granted to the first author and the National Counsel of Technological and Scientific Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq) for the productivity scholarship granted to the other authors. We are also grateful to the Chemical Ecology Laboratory of Embrapa Soja, where the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Rupesh Kariyat.
Rights and permissions
About this article
Cite this article
Vieira, S.S., Lourenção, A.L., da Graça, J.P. et al. Biological aspects of Bemisia tabaci biotype B and the chemical causes of resistance in soybean genotypes. Arthropod-Plant Interactions 10, 525–534 (2016). https://doi.org/10.1007/s11829-016-9458-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-016-9458-4