Abstract
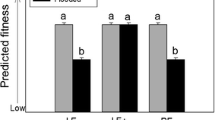
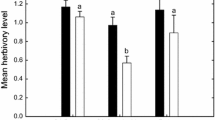
Plant–microbe protection symbioses occur when a symbiont defends its host against enemies (e.g., insect herbivores); these interactions can have important influences on arthropod abundance and composition. Understanding factors that generate context-dependency in protection symbioses will improve predictions on when and where symbionts are most likely to affect the ecology and evolution of host species and their associated communities. Of particular relevance are changes in abiotic contexts that are projected to accompany global warming. For example, increased drought stress can enhance the benefits of fungal symbiosis in plants, which may have multi-trophic consequences for plant-associated arthropods. Here, we tracked colonization of fungal endophyte-symbiotic and aposymbiotic Poa autumnalis (autumn bluegrass) by Rhopalosiphum padi (bird-cherry-oat aphids) and their parasitoids (Aphelinus sp.) following manipulations of soil water levels. Endophyte symbiosis significantly reduced plant colonization by aphids. Under low water, symbiotic plants also supported a significantly higher proportion of aphids that were parasitized by Aphelinus and had higher above-ground biomass than aposymbiotic plants, but these endophyte-mediated effects disappeared under high water. Thus, the multi-trophic consequences of plant-endophyte symbiosis were contingent on the abiotic context, suggesting the potential for complex responses in the arthropod community under future climate shifts.



Similar content being viewed by others
References
Afkhami ME, Rudgers JA (2009) Endophyte-mediated resistance to herbivores depends on herbivore identity in the wild grass Festuca subverticillata. Environ Entomol 38:1086–1095
Arimura G, Matsui K, Takabayashi J (2009) Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol 50:911–923
Bacon CW, White JF Jr (1994) Stains, media, and procedures for analyzing endophytes. In: Bacon CW, White JF Jr (eds) Biotechnology of endophytic fungi of grasses. CRC Press, Boca Raton, pp 47–56
Barkworth ME, Capels KM, Long S, Anderton LK, Piep MB (eds) (2006) Flora of North America volume 24: North of Mexico: Magnoliophyta: Commelinidae (in part): Poaceae, part 1. Oxford University Press, New York, NY
Bazely DR, Vicari M, Emmerich S, Filip L, Lin D, Inman A (1997) Interactions between herbivores and endophyte-infected Festuca rubra from the Scottish islands of St. Kilda, Benbecula and Rum. J App Ecol 34:847–860
Belesky DP, Stringer WC, Plattner RD (1989) Influence of endophyte and water regime upon tall fescue accessions. 2. Pyrrolizidine and ergopeptine alkaloids. Ann Bot 64:343–349
Bieri APS, Härri SA, Vorburger C, Müller CB (2009) Aphid genotypes vary in their response to the presence of fungal endosymbionts in host plants. J Evol Biol 22:1775–1780
Bronstein JL (1994) Conditional outcomes in mutualistic interactions. Trends Ecol Evol 9:214–217
Bronstein JL (1998) The contribution of ant-plant protection studies to our understanding of mutualism. Biotropica 30:150–161
Bultman TL, Bell GD (2003) Interaction between fungal endophytes and environmental stressors influences plant resistance to insects. Oikos 103:182–190
Bultman TL, Borowicz KL, Schneble RM, Coudron TA, Bush LP (1997) Effect of a fungal endophyte on the growth and survival of two Euplectrus parasitoids. Oikos 78:170–176
Bush LP, Fannin FF, Siegel MR, Dahlman DL, Burton HR (1993) Chemistry, occurrence and biological effects of saturated pyrrolizidine alkaloids associated with endophyte grass interactions. Agric Ecosyst Environ 44:81–102
Carver M, Sullivan DJ, Niemczyk E, Dixon AFG (1988) Encapsulative defense reactions of aphids (Hemiptera: Aphididae) to insect parasitoids (Hymenoptera: Aphidiidae and Aphelinidae) (minireview). Ecology and effectiveness of aphidophaga. Proceedings of an international symposium, held at Teresin, Poland, August 31–September 5, 1987, pp 299–303
Carver M, Woolcock LT (1985) Interactions between Acrythosiphon kondoi (Homoptera, Aphidoidea) and Aphelinus asychis (Hymenoptera, Chalcidoidea) and other parasites and hosts. Entomophaga 30:193–198
Cassell DL (2002) A randomization-test wrapper for SAS PROCs. In: Inc SI (ed) Proceedings of the twenty-seventh annual SAS users group international conference. SAS Institute Inc., Cary
Chaneton EJ, Omacini M (2007) Bottom-up cascades induced by fungal endophytes in multitrophic systems. In: Ohgushi T, Craig TP, Price PW (eds) Ecological communities: plant mediation in indirect interaction webs, 1st edn. Cambridge University Press, Cambridge, pp 164–187
Cheplick GP, Faeth SH (2009) Ecology and evolution of grass-endophyte symbiosis. Oxford University Press, Oxford
Clay K (1990) Fungal endophytes of grasses. Annu Rev Ecol Syst 21:275–297
Clay K (1996) Interactions among fungal endophytes, grasses and herbivores. Res Popul Ecol 38:191–201
Clay K, Schardl C (2002) Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am Nat 160:S99–S127
Clay K, Holah J, Rudgers JA (2005) Herbivores cause a rapid increase in hereditary symbiosis and alter plant community composition. Proc Natl Acad Sci USA 102:12465–12470
Clement SL, Elberson LR, Bosque-Perez NA, Schotzko DJ (2005) Detrimental and neutral effects of wild barley—Neotyphodium fungal endophyte associations on insect survival. Entomol Exp Appl 114:119–125
Cohen JE, Jonsson T, Müller CB, Godfray HCJ, Savage VM (2005) Body sizes of hosts and parasitoids in individual feeding relationships. Proc Natl Acad Sci USA 102:684–689
Crawford KM, Land JM, Rudgers JA (2010) Fungal endophytes of native grasses decrease insect herbivore preference and performance. Oecologia 164:431–444
de Sassi C, Müller CB, Krauss J (2006) Fungal plant endosymbionts alter life history and reproductive success of aphid predators. Proc R Soc Lond B Biol Sci 273:1301–1306
Edgington ES (1987) Randomization tests, 2nd edn. Marcel Dekker, New York
Faeth SH, Bultman TL (2002) Endophytic fungi and interactions among host plants, herbivores, and natural enemies. In: Tscharntke T, Hawkins BA (eds) Multitrophic level interactions. Cambridge University Press, Cambridge, pp 89–123
Finkes LK, Cady AB, Mulroy JC, Clay K, Rudgers JA (2006) Plant-fungus mutualism affects spider composition in successional fields. Ecol Lett 9:347–356
Francke DL, Harmon JP, Harvey CT, Ives AR (2008) Pea aphid dropping behavior diminishes foraging efficiency of a predatory ladybeetle. Entomol Exp Appl 127:118–124
Gao F, Zhu SR, Sun YC, Du L, Parajulee M, Kang L, Ge F (2008) Interactive effects of elevated CO2 and cotton cultivar on tri-trophic interaction of Gossypium hirsutum, Aphis gossyppii, and Propylaea japonica. Environ Entomol 37:29–37
Gonthier DJ, Sullivan TJ, Brown KL, Wurtzel B, Lawal R, VandenOever K, Buchan Z, Bultman TL (2008) Stroma-forming endophyte Epichloë glyceriae provides wound-inducible herbivore resistance to its grass host. Oikos 117:629–633
Gould FW (1975) The grasses of Texas. Texas A&M University Press, College Station, TX
Gregory PJ, Johnson SN, Newton AC, Ingram JSI (2009) Integrating pests and pathogens into the climate change/food security debate. J Exp Bot 60:2827–2838
Grewal SK, Grewal PS, Gaugler R (1995) Endophytes of fescue grasses enhance susceptibility of Popillia japonica larvae to an entomopathogenic nematode. Entomol Exp Appl 74:219–224
Gundel PE, Batista WB, Texeira M, Martinez-Ghersa MA, Omacini M, Ghersa CM (2008) Neotyphodium endophyte infection frequency in annual grass populations: relative importance of mutualism and transmission efficiency. Proc R Soc Lond B Biol Sci 275:897–905
Haine ER (2008) Symbiont-mediated protection. Proc R Soc Lond B Biol Sci 275:353–361
Härri SA, Krauss J, Müller CB (2008a) Fungal endosymbionts of plants reduce lifespan of an aphid secondary parasitoid and influence host selection. Proc R Soc Lond B Biol Sci 275:2627–2632
Härri SA, Krauss J, Müller CB (2008b) Natural enemies act faster than endophytic fungi in population control of cereal aphids. J Anim Ecol 77:605–611
Härri SA, Krauss J, Müller CB (2008c) Trophic cascades initiated by fungal plant endosymbionts impair reproductive performance of parasitoids in the second generation. Oecologia 157:399–407
Härri SA, Krauss J, Müller CB (2009) Extended larval development time for aphid parasitoids in the presence of plant endosymbionts. Ecol Entomol 34:20–25
Hatano E, Kunert G, Michaud JP, Weisser WW (2008) Chemical cues mediating aphid location by natural enemies. Eur J Entomol 105:797–806
Hoover JK, Newman JA (2004) Tritrophic interactions in the context of climate change: a model of grasses, cereal aphids and their parasitoids. Glob Chang Biol 10:1197–1208
IPCC (2007) Climate change 2007: the physical science basis. Summary for policy makers. Intergovernmental Panel on Climate Change Geneva, Switzerland, p 18
Jallow MFA, Dugassa-Gobena D, Vidal S (2008) Influence of an endophytic fungus on host plant selection by a polyphagous moth via volatile spectrum changes. Arthropod-Plant Interactions 2:53–62
Kannadan S, Rudgers JA (2008) Endophyte symbiosis benefits a rare grass under low water availability. Funct Ecol 22:706–713
Lehtonen P, Helander M, Saikkonen K (2005) Are endophyte-mediated effects on herbivores conditional on soil nutrients? Oecologia 142:38–45
Lewis GC, Ravel C, Naffaa W, Astier C, Charmet G (1997) Occurrence of Acremonium endophytes in wild populations of Lolium spp. in European countries and a relationship between level of infection and climate in France. Ann Appl Biol 130:227–238
Losey JE, Denno RF (1998) The escape response of pea aphids to foliar-foraging predators: factors affecting dropping behaviour. Ecol Entomol 23:53–61
Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40:923–940
Manly BFJ (1991) Randomization and Monte Carlo methods in biology. Chapman and Hall, London
Moon CD, Craven KD, Leuchtmann A, Clement SL, Schardl CL (2004) Prevalence of interspecific hybrids amongst asexual fungal endophytes of grasses. Mol Ecol 13:1455–1467
Novas MV, Collantes M, Cabral D (2007) Environmental effects on grass-endophyte associations in the harsh conditions of south Patagonia. FEMS Microbiol Ecol 61:164–173
Noyes J (1982) Collecting and preserving chalcid wasps (Hymenoptera: Chalcidoidea). J Nat Hist 16:315–334
Oliver KM, Moran NA, Hunter MS (2005) Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc Natl Acad Sci USA 102:12795–12800
Omacini M, Chaneton EJ, Ghersa CM, Müller CB (2001) Symbiotic fungal endophytes control insect host-parasite interaction webs. Nature 409:78–81
Rodriguez RJ, White JF, Arnold AE, Redman RS (2009) Fungal endophytes: diversity and functional roles. New Phytol 182:314–330
Rudgers JA, Afkhami ME, Rua MA, Davitt AJ, Hammer S, Huguet VM (2009) A fungus among us: broad patterns of endophyte distribution in the grasses. Ecology 90:1531–1539
Rudgers JA, Clay K (2007) Endophyte symbiosis with tall fescue: how strong are the impacts on communities and ecosystems? Fungal Biol Rev 21:107–124
Rudgers JA, Clay K (2008) An invasive plant-fungal mutualism reduces arthropod diversity. Ecol Lett 11:831–840
Rudgers JA, Swafford AL (2009) Benefits of a fungal endophyte in Elymus virginicus decline under drought stress. Basic Appl Ecol 10:43–51
Rudgers JA, Davitt AJ, Clay K, Gundel P, Omacini M (2010) Searching for evidence against the mutualistic nature of hereditary symbiosis: a comment on Faeth (2009). American Naturalist (in press)
Sachs JL, Mueller UG, Wilcox TP, Bull JJ (2004) The evolution of cooperation. Q Rev Biol 79:135–160
Saona NM, Albrectsen BR, Ericson L, Bazely DR (2010) Environmental stresses mediate endophyte-grass interactions in a boreal archipelago. J Ecol 98:470–479
Schardl CL, Grossman RB, Nagabhyru P, Faulkner JR, Mallik UP (2007) Loline alkaloids: currencies of mutualism. Phytochemistry 68:980–996
Siegel MR, Latch GCM, Bush LP, Fannin FF, Rowan DD, Tapper BA, Bacon CW, Johnson MC (1990) Fungal endophyte-infected grasses: alkaloid accumulation and aphid response. J Chem Ecol 16:3301–3316
Villagra CA, Ramirez CC, Niemeyer HM (2002) Antipredator responses of aphids to parasitoids change as a function of aphid physiological state. Anim Behav 64:677–683
White JF Jr, Torres MS (eds) (2009) Defensive mutualism in microbial symbiosis. CRC Press, Boca Raton, FL
Wilkinson HH, Siegel MR, Blankenship JD, Mallory AC, Bush LP, Schardl CL (2000) Contribution of fungal loline alkaloids to protection from aphids in a grass-endophyte mutualism. Mol Plant Microbe Interact 13:1027–1033
Yue Q, Wang CL, Gianfagna TJ, Meyer WA (2001) Volatile compounds of endophyte-free and infected tall fescue (Festuca arundinacea Schreb.). Phytochemistry 58:935–941
Züst T, Härri SA, Müller CB (2008) Endophytic fungi decrease available resources for the aphid Rhopalosiphum padi and impair their ability to induce defenses against predators. Ecol Entomol 33:80–85
Acknowledgments
This work was funded by the Godwin Assistant Professorship and NSF-DEB#054278 to J.A.R. and by the Rice Century Scholars Fund to K.M.Y. We would like to thank Alex Gorischek, Liz Seifert, and Sami Hammer for assistance with the experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Robert Glinwood.
Rights and permissions
About this article
Cite this article
Yule, K.M., Woolley, J.B. & Rudgers, J.A. Water availability alters the tri-trophic consequences of a plant-fungal symbiosis. Arthropod-Plant Interactions 5, 19–27 (2011). https://doi.org/10.1007/s11829-010-9112-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-010-9112-5