Abstract
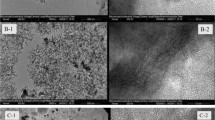
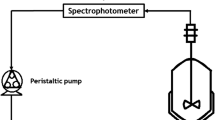
The Magnetic nanoporous material Fe/MCM-41 was prepared, and its physical characterization studied, to determine the effect of its properties on separation efficiency of methyl orange (MO) from wastewater by adsorption process. The experimental results were analyzed for both adsorbent mesoporous material samples, MCM-41 and magnetic Fe/MCM-41, in order to select the best operating conditions for the different studied parameters, which are: constant temperature (20 °C), pH: (2) adsorbent dosage (0.03 gm), contact time (10minute) and concentrations (30mg/L). The results demonstrate that the adsorption processes can be well fitted by the Langmuir isotherm model for pure MCM-41, with a correlation coefficient of (0.999), and fitted by the Freundlich isotherm model for magnetic Fe/ MCM-41, with a correlation coefficient of (0.994). The adsorption kinetics of MO on to MCM-41 and Fe/MCM-41 are well described by a pseudo-second-order kinetic model.
Similar content being viewed by others
References
A. Rodríguez, J. García, G. Ovejero and M. Mestanza, J. Hazard. Mater., 172, 1311 (2012).
E. Forgacs, T. Cserháti and G. Oros, Environ. Int., 30, 953 (2009).
Q. F. Alsalhy, T. M. Albyati and M.A. Zablouk, Chem. Eng. Commun., 200(1), 1 (2013).
T. M. Albayati and A. M. Doyle, Adsorpt. Sci. Technol., 31(5), 459 (2013).
T. M. Albayati and A. M. Doyle., Chem.: Bulgarian J. Sci. Education, 23(1), 105 (2014).
T. M. Albayati, Part. Sci. Technol., 32(6), 616 (2014).
A. A. Sabri, T. M. Albayati and R. A. Alazawi, Korean J. Chem. Eng., 32(9), 1835 (2015).
S. Babel and T. A. Kurniawan, J. Hazard. Mater., 97, 219 (2003).
V. K. Gupta, J. Environ. Manage., 90, 2313 (2009).
A. H. Lu, W. C. Li., A. Kiefer, W. Schmidt, E. Bill, G. Fink and F. Schuth, J. Am. Chem. Soc., 126, 8616 (2004).
P. Wu, J. Zhu and Z. Xu, Adv. Funct. Mater., 14, 345 (2004).
S. Huang, P. Yang, Z. Cheng, C. Li., Y. Fan, D. Kong and J. Lin, J. Phys. Chem. C, 112, 7130 (2008).
S. Giri, B. G. Trewyn, M. P. Stellmaker and V. S. Lin, Angew. Chem. Int. Ed., 44, 5038 (2005).
Y. Deng, D. Qi, C. Deng, X. Zhang and D. Zhao, J. Am. Chem. Soc., 130, 28 (2008).
Y. Xingbin, C. Jiangtao, X. Qunji and M. Philippe, Micropor. Mesopor. Mater., 135, 137 (2010).
H. Chen and Y. Wang, Ceram. Int., 28, 541 (2002).
T.M. Albayati and A.M. Doyle, J. Nanopart. Res., 17(2), 109 (2015).
I. Langmuir, J. Am. Chem. Soc., 38(11), 2221 (1916).
W. J. Weber and R. K. Chakravorti, J. Am. Inst. Chem. Eng., 20, 228 (1974).
M. Chen, Y. Chen and G.W. Diao, J. Chem. Eng. Data, 55, 5109 (2010).
H.M. Freundlich, Colloid Capillary Chemistry, 3(12), 1454 (1926).
H.M. Freundlich, Z. Phys. Chem., 57A, 385 (1906).
L. Yuhan, L. Mingce, H. Peidong, C. Ya and H. Juwei, J. Hazard. Mater., 264, 195 (2014).
C. Xinqing, F.L. Koon and L.Y. King, Chem. Eng. J., 172, 728 (2011).
U. Irina, S. Alexandru and V. Aurelia, J. Colloid Interface Sci., 377, 184 (2012).
B. S. Liu, D.F. Xu, J.X. Chu, W. Liu and C.T. Au, Energy Fuels, 21, 250 (2007).
C. Xinqing, A. Manuel and L.Y. King, Catal. Today, 204, 140 (2013).
Z. Weijia, S. Z. Wenhe, W. Yingjun, Z. Hongshi, L. Zhengmao and Y. Shunpu, J. Magn. Magn. Mater., 321, 1025 (2009).
L. Yujin, S. Kunyan, L. Rongzhan, Z. Xin, Z. Yang, L. Hongchao and X. Yanzhi, Energy Procedia, 61, 863 (2012).
S. Rengaraj, C. K. Joo, Y. Kim and J. Yi, J. Hazard. Mater., 102(2-3), 257 (2003).
J. Tang, Z. f. Yang and Y. J. Yi, Procedia Environ. Sci., 13, 2179 (2012).
Q. Meng, X. Zhang, C. He, P. Zhou, W. Su and C. Duan, Talanta, 84, 53 (2011).
H. Ruihua, L. Qian, H. Jie and Y. Bingchao, Arabian J. Chem. (2013), DOI:10.1016/j.arabjc.2013.05.017.
T. Fang-Chang, M. Ning, C. Tai-Chin, T. Lung-Chang, S. Jing-Jing, A. Yue Xia, T. Jiang, S. Shuenn-Kung and C. Fu-Sheng, J. Water Process Eng., 1, 2 (2014).
L. Tonghao, L. Yanhui, D. Qiuju, S. Jiankun, J. Yuqin, Y. Guangming, W. Zonghua, X. Yanzhi, Z. Wei, W. Kunlin, Z. Hongwei and W. Dehai, Colloids Surf., B: Bio Interfaces, 90, 197 (2012).
Y. Yunjin, H. Bing, X. Feifei and C. Xiaofeng, Chem. Eng. J., 170, 82 (2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Albayati, T.M., Alwan, G.M. & Mahdy, O.S. High performance methyl orange capture on magnetic nanoporous MCM-41 prepared by incipient wetness impregnation method. Korean J. Chem. Eng. 34, 259–265 (2017). https://doi.org/10.1007/s11814-016-0231-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-016-0231-2