Abstract
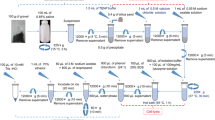
The biologic activated carbon (BAC) process is widely used in drinking water treatments. A comprehensive molecular analysis of the microbial community structure provides very helpful data to improve the reactor performance. However, the bottleneck of deoxyribonucleic acid (DNA) extraction from BAC attached biofilm has to be solved since the conventional procedure was unsuccessful due to firm biomass attachment and adsorption capacity of the BAC granules. In this study, five pretreatments were compared, and adding skim milk followed by ultrasonic vibration was proven to be the optimal choice. This protocol was further tested using the vertical BAC samples from the full-scale biofilter of Pinghu Water Plant. The results showed the DNAyielded a range of 40 μg·g−1 BAC (dry weight) to over 100 μg·g−1 BAC (dry weight), which were consistent with the biomass distribution. All results suggested that the final protocol could produce qualified genomic DNA as a template from the BAC filter for downstream molecular biology researches.
Similar content being viewed by others
References
Nishijima W, Kim W, Shoto E, Okada M. The performance of an ozonation-biological activated carbon process under long term operation. Water Science and Technology, 1998, 38(6): 163–169
Graham N J D. Removal of humic substances by oxidation/biofiltration processes-A review. Water Science and Technology, 1999, 40(9): 141–148
Xu B, Gao N Y, Sun X F, Xia S J, Simonnot M O, Causserand C, Rui M, Wu H H. Characteristics of organic material in Huangpu River and treatability with the O3-BAC process. Separation and Purification Technology, 2007, 57(2): 348–355
Kim W, Nishijima W, Baes A, Okada M. Micropollutant removal with saturated biological activated carbon(BAC) in ozonation-BAC process. Water Science and Technology, 1997, 36(12): 283–298
Cheng L, Gao N Y, Cai Y L. Influence of the disinfectants on the biological stability of the drinking water. Journal of Xi’an University of Architecture & Technology (Natural Science Edition), 2007, 39(2): 263–267
Sirotkin A, Koshkina L, Ippolitov K. The BAC-process for treatment of waste water containing non-ionogenic synthetic surfactants. Water Research, 2001, 35(13): 3265–3271
Lin W, Weber A. Aerobic biological activated carbon (BAC) treatment of a phenolic wastewater. Environment and Progress, 1992, 11(2): 145–154
Chung Y C, Lin Y Y, Tseng C P. Operational characteristics of effective removal of H2S and NH3 waste gases by activated carbon biofilter. Journal of the Air & Waste Management Association, 2004, 54(4): 450–458
Duan H, Yan R, Koe L C. Investigation on the mechanism of H2S removal by biological activated carbon in a horizontal biotrickling filter. Applied Microbiology and Biotechnology, 2005, 69(3): 350–357
Lin C K, Tsai T Y, Liu J C, Chen M C. Enhanced biodegradation of petrochemical wastewater using ozonation and BAC advanced treatment system. Water Research, 2001, 35(3): 699–704
Kim W H, Nishijima W, Shoto E, Okada M. Pilot plant study on ozonation and biological activated carbon process for drinking water treatment. Water Science and Technology, 1997, 35(8): 21–28
Simpson D R. Biofilm processes in biologically active carbon water purification. Water Research, 2008, 42(12): 2839–2848
Rompré A, Servais P, Baudart J, de-Roubin M, Laurent P. Detection and enumeration of coliforms in drinking water: current methods and emerging approaches. Journal of Microbiological Methods, 2002, 49(1): 31–54
DeVet W W J M, Dinkla I J T, Muyzer G, Rietveld L C, van Loosdrecht M C M. Molecular characterization of microbial populations in groundwater sources and sand filters for drinking water production. Water Research, 2009, 43(1): 182–194
Fonseca A C, Summers R S, Hernandez M T. Comparative measurements of microbial activity in drinking water biofilters. Water Research, 2001, 35(16): 3817–3824
Steele J, Ozis F, Fuhrman J A, Devinny J S. Structure of microbial communities in ethanol biofilters. Chemical Engineering Journal, 2005, 113(2–3): 135–143
Liu X L, Liu W J. Microbial community structure in bio-ceramics and biological activated carbon analyzed by PCR-SSCP technique. Huan Jing Ke Xue, 2007, 28(4): 924–928 (in Chinese)
Kyotani T. Control of pore structure in carbon. Carbon, 2000, 38(2): 269–286
Li L, Quinlivan P, Knappe D. Effects of activated carbon surface chemistry and pore structure on the adsorption of organic contaminants from aqueous solution. Carbon, 2002, 40(12): 2085–2100
Frostegard A, Courtois S, Ramisse V, Clerc S, Bernillon D, Le Gall F, Jeannin P, Nesme X, Simonet P. Quantification of bias related to the extraction of DNA directly from soils. Applied and Environmental Microbiology, 1999, 65(12): 5409–5420
Volossiouk T, Robb E J, Nazar R N. Direct DNA extraction for PCR-mediated assays of soil organisms. Applied and Environmental Microbiology, 1995, 61(11): 3972–3976
García-Pedrajas M D, Bainbridge B W, Heale J B, Perez-Artes E, Jimenez-Diaz R M. A simple PCR-based methods for the detection of the chickpea-wilt pathogen Fusarium oxysporum f.sp.ciceria in artificial and natural soils. European Journal of Plant Pathology, 1999, 105(3): 251–259
Takada-Hoshino Y, Matsumoto N. An improved DNA extraction method using skim milk from soils that strongly adsorb DNA. Microbes and Environments, 2004, 19(1): 13–19
Ikeda S, Tsurumaru H, Wakai S, Noritake C, Fujishiro K, Akasaka M, Ando K. Evaluation of the effects of different additives in improving the DNA extraction yield and quality from Andosol. Microbes and Environments, 2008, 23(2): 159–166
Zhou J, BrunsM A, Tiedje J M. DNA recovery from soils of diverse composition. Applied and Environmental Microbiology, 1996, 62(2): 316–322
Buesing N, Gessner M O. Comparison of detachment procedures for direct counts of bacteria associated with sediment particles, plant litter and epiphytic biofilms. Aquatic Microbial Ecologys, 2002, 27(1): 29–36
Frey J C, Angert E R, Pell A N. Assessment of biases associated with profiling simple, model communities using terminal-restriction fragment length polymorphism-based analyses. Journal of Microbiological Methods, 2006, 67(1): 9–19
Griffiths R I, Whiteley A S, O’Donnell A G, Bailey M J. Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Applied and Environmental Microbiology, 2000, 66(12): 5488–5491
Uzel A, Ozdemir G. Metal biosorption capacity of the organic solvent tolerant Pseudomonas fluorescens TEM08. Bioresource Technology, 2009, 100(2): 542–548
Yu X, Zhang X, Wang Z. Biomass determination using Lipid-P method for drinking water biological treatment. Water and Wastewater Engineering, 2002, 28(5): 1–5 (in Chinese)
Wang J Z, Summers R S, Miltner R J. Biofiltration Performance: Part 1, Relationship to Biomass. Journal AWWA, 1995, 87(12): 55–63
Ogram A V, Mathot M L, Harsh J B, Boyle J, Pettigrew C A. Effects of DNA polymer length on its adsorption to soils. Applied and Environmental Microbiology, 1994, 60(2): 393–396
Paget E, Monrozier L J, Simonet P. Adsorption of DNA on clay minerals: protection against Dnase I and influence on gene transfer. FEMS Microbiology Letters, 1992, 97(1–2): 31–39
Ogram A, Sayler G S, Barkay T. The extraction and purification of microbial DNA from sediments. Journal of Microbiological Methods, 1987, 7(2–3): 57–66
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, S., Wei, B., Yu, X. et al. Development of pretreatment protocol for DNA extraction from biofilm attached to biologic activated carbon (BAC) granules. Front. Environ. Sci. Eng. China 4, 459–465 (2010). https://doi.org/10.1007/s11783-010-0249-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11783-010-0249-3