Abstract
Background
Neoadjuvant chemotherapy (NAC) with CF (cisplatin/5-FU) was demonstrated to improve survival of clinical stage II/III (cStage II/III) esophageal squamous cell carcinoma (ESCC), however prognostic outcome remains unsatisfactory. We have recently reported preliminary potentiality of short-term survival benefit by NAC with DCF (docetaxel/cisplatin/5-FU).
Patients and methods
Thirty-eight ESCC patients who underwent DCF NAC between 2009 and 2012 were investigated for prognosis with a median follow-up period of 49 months as compared to those with CF NAC.
Results
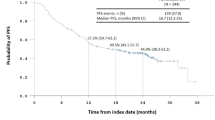
(1) ESCC patients with DCF NAC showed 66 % of 3-year progression-free survival (PFS), which is significantly superior to that of CF NAC (38 %) (p = 0.018). ESCC patients with DCF NAC showed 79 % of 3-year overall survival (OS), which is marginally significantly superior to that of CF NAC (65 %) (p = 0.093). (2) The multivariate Cox proportional hazards model revealed that DCF NAC was an independent prognostic factor for PFS (p = 0.0013) and OS (p = 0.047), respectively, when adjusted for patient sex, age, cT, cN, and preoperative borderline resectability. (3) Patients with more advanced stage were rather frequently included in DCF NAC than in CF NAC, however there was no significant difference. Nevertheless, propensity score (PS) to predict DCF NAC was significantly higher than CF NAC (p = 0.019). (4) Both NAC and PS were again applied to the multivariate Cox proportional hazards model, and DCF NAC was the only remnant prognostic indicator for PFS (p = 0.0044) and OS (p = 0.063).
Conclusion
Prognosis may be significantly improved in cStage II/III ESCC patients who underwent DCF NAC than those with CF NAC.


Similar content being viewed by others
References
International Agency for Research on Cancer WHO. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence; 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18–29.
Scarpa M, Valente S, Alfieri R, Cagol M, Diamantis G, Ancona E, Castoro C. Systematic review of health-related quality of life after esophagectomy for esophageal cancer. World J Gastroenterol. 2011;17:4660–74.
Law S, Wong J. Two-field dissection is enough for esophageal cancer. Dis Esophagus. 2001;14:98–103.
Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H, Tilanus HW, van Lanschot JJ. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–9.
Ooki A, Yamashita K, Kobayashi N, Katada N, Sakuramoto S, Kikuchi S, Watanabe M. Lymph node metastasis density and growth pattern as independent prognostic factors in advanced esophageal squamous cell carcinoma. World J Surg. 2007;31:2184–91.
Yamashita K, Katada N, Moriya H, Hosoda K, Sakuramoto S, Kikuchi S, Watanabe M. Multimodality treatment and prognosis in esophageal squamous cell carcinoma requiring esophagectomy. Hepatogastroenterology. 2014;61:1042–8.
Ando N, Iizuka T, Ide H, Ishida K, Shinoda M, Nishimaki T, Takiyama W, Watanabe H, Isono K, Aoyama N, Makuuchi H, Tanaka O, Yamana H, Ikeuchi S, Kabuto T, Nagai K, Shimada Y, Kinjo Y, Fukuda H, Japan Clinical Oncology Group. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study—JCOG9204. J Clin Oncol. 2003;21:4592–6.
Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki HAN, Kurita A, Ikeda K, Kanda T, Tsujinaka T, Nakamura K, Fukuda H. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68–74.
Katada N, Yamashita K, Katada C, Moriya H, Hosoda K, Mieno H, Higuchi K, Komoroi S, Ishiyama H, Hayakawa K, Sugawara M, Tanabe S, Koizumi W, Kikuchi S, Watanabe M. Neoadjuvant chemotherapy using concurrent docetaxel/CDDP/5-FU (DCF) in esophageal squamous cell carcinoma and its short-term prognosis. Esophagus. 2014;11:173–81.
Shah MA, Jhawer M, Ilson DH, Lefkowitz RA, Robinson E, Capanu M, Kelsen DP. Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol. 2011;29:868–74.
Tanaka Y, Yoshida K, Sanada Y, Osada S, Yamaguchi K, Takahashi T. Biweekly docetaxel, cisplatin, and 5-fluorouracil (DCF) chemotherapy for advanced esophageal squamous cell carcinoma: a phase I dose-escalation study. Cancer Chemother Pharmacol. 2010;66:1159–65.
Yamasaki M, Miyata H, Tanaka K, Shiraishi O, Motoori M, Peng YF, Yasuda T, Yano MSH, Mori M, Doki Y. Multicenter phase I/II study of docetaxel, cisplatin and fluorouracil combination chemotherapy in patients with advanced or recurrent squamous cell carcinoma of the esophagus. Oncology. 2011;80:307–13.
Bissery MC, Vrignaud P, Lavelle F. Preclinical profile of docetaxel (taxotere): efficacy as a single agent and in combination. Semin Oncol. 1995;22:3–16.
Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM, Cullen K, Ervin TJ, Murphy BA, Raez LE, Cohen RB, Spaulding M, Tishler RB, Roth B, Viroglio RC, Venkatesan V, Romanov I, Agarwala S, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15.
Ferri LE, Ades S, Alcindor T, Chasen M, Marcus V, Hickeson M, Artho G, Thirlwell MP. Perioperative docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: a multicenter phase II trial. Ann Oncol. 2012;23:1512–7.
Yokota T, Hatooka S, Ura T, Abe T, Takahari D, Shitara K, Nomura M, Kondo C, Mizota AYY, Shinoda M, Muro K. Docetaxel plus 5-fluorouracil and cisplatin (DCF) induction chemotherapy for locally advanced borderline-resectable T4 esophageal cancer. Anticancer Res. 2011;31:3535–41.
Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, Okawara G, Rosenthal SA, Kelsen DP. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–74.
Hara H, Tahara M, Daiko H, Kato K, Igaki H, Kadowaki S, Tanaka Y, Hamamoto Y, Matsushita H, Nagase M, Hosoya Y. Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1455–60.
Japanese Society for Esophageal Disease. Guidelines for the clinical and pathological studies on carcinoma of the esophagus. 10th ed. Kanehara; 2002.
Clavien PA, Barkun J, de Oliveira ML. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
Higuchi K, Komori S, Tanabe S, Katada C, Azuma M, Ishiyama H, Sasaki T, Ishido KKN, Hayakawa K, Koizumi W, Kitasato Digestive Disease and Oncology Group. Definitive chemoradiation therapy with docetaxel, cisplatin, and 5-fluorouracil (DCF-R) in advanced esophageal cancer: a phase 2 trial (KDOG 0501-P2). Int J Radiat Oncol Biol Phys. 2014;89:872–9.
Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimer J, Estes N, Haller DG, Ajani J, Kocha W, Minsky BD, Roth JA. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979–84.
Nakamura K, Kato K, Igaki H, Ito Y, Mizusawa J, Ando N, Udagawa H, Tsubosa Y, Daiko H, Hironaka S, Fukuda H, Kitagawa Y, Japan Esophageal Oncology Group/Japan, Clinical Oncology Group. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol. 2013;43:752–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of authors have any financial conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11748_2016_626_MOESM1_ESM.pptx
Fig. S1 PFS of cStage II/III ESCC according to various clinical factors. (a) Sex, (b) Age, (c) cT, (d) borderline resectability, and (e) cN (PPTX 70 kb)
11748_2016_626_MOESM2_ESM.pptx
Fig. S2 OS of cStage II/III ESCC according to various clinical factors. (a) Sex, (b) Age, (c) cT, (d) borderline resectability, and (e) cN (PPTX 70 kb)
Rights and permissions
About this article
Cite this article
Yamashita, K., Katada, N., Moriya, H. et al. Neoadjuvant chemotherapy of triplet regimens of docetaxel/cisplatin/5-FU (DCF NAC) may improve patient prognosis of cStage II/III esophageal squamous cell carcinoma-propensity score analysis. Gen Thorac Cardiovasc Surg 64, 209–215 (2016). https://doi.org/10.1007/s11748-016-0626-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-016-0626-3