Abstract
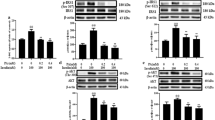
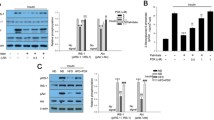
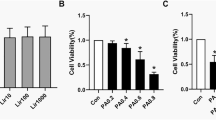
Alteration of lipid metabolism is an important mechanism for the treatment of insulin resistance. PGC-1α, a key regulator of mitochondrial biogenesis and function, plays an important role in the improvement of insulin sensitivity by increasing fatty acids β-oxidation. In the present study, the effects of epigallocatechin-3-gallate (EGCG), an anti-obesity agent and enhancer of lipid catabolism, on PGC-1α protein expression was examined and compared with anti-diabetic drug rosiglitazone (RGZ). After differentiation of C2C12 myoblasts to myotubes, insulin resistance was induced by palmitate treatment. Then the expression of the PGC-1a gene and glucose uptake were evaluated before and after treatment with RGZ and EGCG. Palmitate treatment significantly decreased PGC-1α protein expression in C2C12 cells (P < 0.05). RGZ could restore the expression of PGC-1α in palmitate treated cells (P > 0.05), while EGCG had no significant effect on the expression of this gene (P < 0.05). RGZ and EGCG significantly improved glucose uptake (by 2- and 1.54-fold, respectively) in myotubes treated with palmitate. These data suggest that RGZ and EGCG both exert their anti-diabetic activity by increasing insulin sensitivity, but with different molecular mechanisms. This effect of RGZ, unlike EGCG, is mediated, at least partly, by increasing PGC-1α protein expression.




Similar content being viewed by others
Abbreviations
- RZG:
-
Rosiglitazone
- EGCG:
-
Epigallocatechin-3-gallate
- PGC-1α:
-
Peroxisome proliferator-activated receptor gamma coactivator-1α
- BSA:
-
Bovine serum albumin
- 2-DOG:
-
2-Deoxyglucose
- T2DM:
-
Type 2 diabetes mellitus
- FAs:
-
Fatty acids
References
Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C et al (2007) Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol 293:H2009–H2023
Reaven GM, Chen YD (1996) Insulin resistance, its consequences, and coronary heart disease. Must we choose one culprit? Circulation 93:1780–1783
Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsøe R, Dela F (2007) Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50:790–796
Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S et al (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100:8466–8471
Lowell BB, Shulman GI (2005) Mitochondrial dysfunction and type 2 diabetes. Science 307:384–387
Taylor R (2004) Causation of type 2 diabetes—the Gordian knot unravels. N Engl J Med 350:639–641
He J, Watkins S, Kelley DE (2001) Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes 50:817–823
Kelley DE, He J, Menshikova EV, Ritov VB (2002) Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950
Morino K, Petersen KF, Shulman GI (2006) Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55:S9–S15
Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA et al (2005) A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 54:1926–1933
Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O et al (2005) Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 280:33588–33598
Miller WC, Bryce GR, Conlee RK (1984) Adaptations to a high-fat diet that increase exercise endurance in male rats. J Appl Physiol Respir Environ Exerc Physiol 56:78–83
Simi B, Sempore B, Mayet MH, Favier RJ (1991) Additive effects of training and high-fat diet on energy metabolism during exercise. J Appl Physiol 71:197–203
Richardson DK, Kashyap S, Bajaj M, Cusi K, Mandarino SJ, Finlayson J et al (2005) Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. J Biol Chem 280:10290–10297
Finck BN, Kelly DP (2006) PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 116:615–622
Boden G, Shulman GI (2002) Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 32:14–23
Fonseca V, Rosenstock J, Patwardhan R, Salzman A (2000) Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a randomized controlled trial. JAMA 283:1695–1702
Lebovitz HE, Dole JF, Patwardhan R, Rappaport EB, Freed MI (2001) Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metab 86:280–288
Wolffenbuttel BH, Gomis R, Squatrito S, Jones NP, Patwardhan RN (2000) Addition of low-dose rosiglitazone to sulphonylurea therapy improves glycaemic control in Type 2 diabetic patients. Diabet Med 17:40–47
Zinman B (2001) PPAR gamma agonists in type 2 diabetes: how far have we come in ‘preventing the inevitable’? A review of the metabolic effects of rosiglitazone. Diabetes Obes Metab 3:S34–S43
Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW et al (2002) The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes 51:797–802
Bogacka I, Xie H, Bray GA, Smith SR (2005) Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes 54:1392–1399
Cleasby ME, Dzamko N, Hegarty BD, Cooney GJ, Kraegen EW, Ye JM (2004) Metformin prevents the development of acute lipid-induced insulin resistance in the rat through altered hepatic signaling mechanisms. Diabetes 53:3258–3266
Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M et al (2006) Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 55:120–127
Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WG, Schlattner U et al (2004) Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem 279:43940–43951
Murase T, Haramizu S, Shimotoyodome A, Nagasawa A, Tokimitsu I (2005) Green tea extract improves endurance capacity and increases muscle lipid oxidation in mice. Am J Physiol Regul Integr Comp Physiol 288:R708–R715
Shimotoyodome A, Haramizu S, Inaba M, Murase T, Tokimitsu I (2005) Exercise and green tea extract stimulate fat oxidation and prevent obesity in mice. Med Sci Sports Exerc 37:1884–1892
Wu LY, Juan CC, Ho LT, Hsu YP, Hwang LS (2004) Effect of green tea supplementation on insulin sensitivity in Sprague-Dawley rats. J Agric Food Chem 52:643–648
Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A et al (2007) EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab 292:E1378–E1387
Krentz AJ, Bailey CJ (2005) Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 65:385–411
Yang J, Han Y, Sun H, Chen C, He D, Guo J, Yu C, Jiang B, Zhou L, Zeng C et al (2011) (–)-Epigallocatechin gallate suppresses proliferation of vascular smooth muscle cells induced by high glucose by inhibition of PKC and ERK1/2 signalings. J Agric Food Chem 59:11483–11490
Bradford MM (1970) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Haghani K, Bakhtiyari S, Nouri AM (2012) In vitro study of the differentiation of bone marrow stromal cells into cardiomyocyte-like cells. Mol Cell Biochem 361:315–320
DeFronzo RA, Ferrannini E (1991) Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 14:173–194
Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL et al (1999) Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42:113–116
Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W et al (1999) Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes 48:1113–1119
Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C et al (1999) Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 48:1600–1606
Coll T, Jove M, Rodriguez-Calvo R, Eyre E, Palomer X, Sanchez RM et al (2006) Palmitate-mediated downregulation of peroxisome proliferator-activated receptor-gamma coactivator 1alpha in skeletal muscle cells involves MEK1/2 and nuclear factor-kappaB activation. Diabetes 55:2779–2787
Bakhtiyari S, Meshkani R, Taghikhani M, Larijani B, Adeli K (2010) Protein tyrosine phosphatase-1B (PTP-1B) knockdown improves palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Lipids 45:237–244
Gorgani-Firuzjaee S, Bakhtiyari S, Golestani A, Meshkani R (2012) Leukocyte antigen-related inhibition attenuates palmitate-induced insulin resistance in muscle cells. J Endocrinol 215:71–77
Jove M, Salla J, Planavila A, Cabrero A, Michalik L, Wahli W et al (2004) Impaired expression of NADH dehydrogenase subunit 1 and PPARgamma coactivator-1 in skeletal muscle of ZDF rats: restoration by troglitazone. J Lipid Res 45:113–123
Parvaneh L, Meshkani R, Bakhtiyari S, Mohammadtaghvaie N, Gorganifiruzjaee S, Taheripak G et al (2010) Palmitate and inflammatory state additively induce the expression of PTP1B in muscle cells. Biochem Biophys Res Commun 396:467–471
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V et al (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124
Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O et al (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418:797–801
Manco M, Mingrone G, Greco AV, Capristo E, Gniuli D, De Gaetano A et al (2000) Insulin resistance directly correlates with increased saturated fatty acids in skeletal muscle triglycerides. Metabolism 49:220–224
Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ et al (2001) Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci USA 98(7):3820–3825
Willson TM, Cobb JE, Cowan DJ, Wiethe RW, Correa ID, Prakash SR et al (1996) The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. J Med Chem 39:665–668
Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R et al (2004) Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes 53:1052–1059
Guri AJ, Hontecillas R, Si H, Liu D, Bassaganya-Riera J (2007) Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets. Clin Nutr 26:107–116
Hontecillas R, O’Shea M, Einerhand A, Diguardo M, Bassaganya-Riera J (2009) Activation of PPAR gamma and alpha by punicic acid ameliorates glucose tolerance and suppresses obesity-related inflammation. J Am Coll Nutr 28:184–195
Salam NK, Huang TH, Kota BP, Kim MS, Li Y, Hibbs DE (2008) Novel PPAR-gamma agonists identified from a natural product library: a virtual screening, induced-fit docking and biological assay study. Chem Biol Drug Des 71:57–70
Liu Y, Zhao B, Mao G, Fang X, Huang Y, Wang N (2013) Epigallocatechin-3-O-gallate, a green tea polyphenol, induces expression of pim-1 kinase via PPARgamma in human vascular endothelial cells. Cardiovasc Toxicol 13:391–395
Ahn J, Lee H, Kim S, Park J, Ha T (2008) The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun 373:545–549
Collins QF, Liu HY, Pi J, Liu Z, Quon MJ, Cao W (2007) Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J Biol Chem 282:30143–30149
Bollag GE, Roth RA, Beaudoin J, Mochly-Rosen D, Koshland DE Jr (1986) Protein kinase C directly phosphorylates the insulin receptor in vitro and reduces its protein-tyrosine kinase activity. Proc Natl Acad Sci USA 83:5822–5824
Deng YT, Chang TW, Lee MS, Lin JK (2012) Suppression of free fatty acid-induced insulin resistance by phytopolyphenols in C2C12 mouse skeletal muscle cells. J Agric Food Chem 60:1059–1066
Acknowledgments
This project was supported financially by deputy for research and technology of Ilam University of Medical Sciences, Iran (Grant 901009/60). We also thank all volunteers for their participation in the study.
Conflict of interest
All authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Karimfar, M.H., Haghani, K., Babakhani, A. et al. Rosiglitazone, but Not Epigallocatechin-3-Gallate, Attenuates the Decrease in PGC-1α Protein Levels in Palmitate-Induced Insulin-Resistant C2C12 Cells. Lipids 50, 521–528 (2015). https://doi.org/10.1007/s11745-015-4016-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-015-4016-x