Abstract
The aim of this study was to determine the influence of long-term docosahexaenoic acid (DHA) dietary supplementation on the erythrocyte fatty acid profile and oxidative balance in soccer players after training and acute exercise. Fifteen volunteer male athletes (age 20.0 ± 0.5 years) were randomly assigned to a placebo group that consumed an almond-based beverage (n = 6), or to an experimental group that consumed the same beverage enriched with DHA (n = 9) for 8 weeks. Blood samples were taken in resting conditions at the beginning and after 8 weeks of nutritional intervention and training in resting and in post-exercise conditions. Oxidative damage markers (malonyldialdehyde, carbonyl and nitrotyrosine indexes) and the activity and protein level of antioxidant enzymes (catalase, superoxide dismutase, glutathione reductase and peroxidase) were assessed. The results showed that training increased antioxidant enzyme activities in erythrocytes. The experimental beverage increased DHA from 34.0 ± 3.6 to 43.0 ± 3.6 nmol/109 erythrocytes. DHA supplementation increased the catalytic activity of superoxide dismutase from 1.48 ± 0.40 to 10.5 ± 0.35 pkat/109 erythrocytes, and brought about a reduction in peroxidative damage induced by training or exercise. In conclusion, dietary supplementation with DHA changed the erythrocyte membrane composition, provided antioxidant defense and reduced protein peroxidative damage in the red blood cells of professional athletes after an 8-week training season and acute exercise.
Similar content being viewed by others
Introduction
Regular physical activity for a healthy body induces adaptation against elevated oxidant levels by increasing cellular and plasma antioxidant capability [1, 2]. However, with acute exercise, this adaptation is minimal, and antioxidant capability can be overwhelmed by oxidant production [3]. Reactive oxygen species (ROS) comprise both free radical and non-free radical oxygen intermediates such as hydrogen peroxide, superoxide, single oxygen, hydroxyl radical, lipid hydroperoxide and lipid radicals [4], and these species may damage cellular macromolecules through oxidative modification [5]. However, it is well established that ROS, when present at low or moderate levels, act as intracellular redox signaling molecules, as evidenced in HL60 cell cultures stimulated with an acute level of hydrogen peroxide or with a continuous low level production of hydrogen peroxide [6]. Long-term stimulation of the endogenous defense mechanism due to regular physical activity brings about the continuous presence of physiological oxidant stimuli, resulting in antioxidant adaptation and greater protection against oxidative challenges [6]. Moreover, extensive and repetitive exercise performed in extreme environmental conditions may cause muscle damage, due to overtraining, and result in reduced athletic performance [3]. Acute exercise involves a kinetic shift in antioxidant enzymes increasing their activities and gene expression which, however, may not be sufficient to restore an oxidant-antioxidant redox balance [7].
Erythrocytes are highly susceptible to oxidative stress and cell damage [8]. The high polyunsaturated fatty acid (PUFA) content of the erythrocyte membranes together with the high quantity of oxygen and heme iron in the erythrocyte are adequate conditions to produce the peroxidation of PUFA. Erythrocytes generate significant amounts of anion superoxide, hydrogen peroxide, simultaneously to the oxidative reaction of hemoglobin with oxygen [9]. Exercise induces a situation of increased oxygen transport, thereby enhancing the capabilities to produce oxidative damage in lipids of erythrocytes. The inability to repair damaged components caused by ROS can increase the rate of hemolysis. However, the ROS produced in erythrocytes can be eliminated by antioxidant defense system enzymes: catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GRd) and glutathione peroxidase (GPx) [7, 8]; as well as the oxidized/reduced glutathione system, and other low molecular weight antioxidants such as vitamins E and C [10].
Dietary intake of n-3 fatty acids, and specifically supplementation with docosahexaenoic acid (22:6n-3; DHA), increases blood levels of this fatty acid [11–14]. A potential increase of unsaturated fatty acids enhances oxidative susceptibility to producing lipid hydroperoxides and oxidative-derived products. Paradoxically, beneficial effects have been pointed out with a diet rich in n-3 fatty acids for promoting maintenance of the antioxidant status and reduction of oxidative damage [13, 15]. Moreover, the antioxidant effects of a mixture of n-3 PUFA have been reported to work by inhibiting lipid peroxidation in erythrocytes [16]. The effects of DHA supplementation on oxidative stress may depend on resting or post-exercise conditions [17], and on antioxidant intake taken together with DHA [18]. In resting conditions, biomarkers of oxidative stress decrease with DHA treatment [17], whereas in post-exercise conditions, the effect of supplementation on stress oxidative parameters is not clearly known. DHA supplementation may have different effects depending on immune cell type [19, 20]. Peripheral blood mononuclear cells (PBMC) and neutrophils respond to exercise stimuli by increasing the capacity to produce ROS [21, 22]. DHA diet supplementation has been seen to increase the PBMC protein levels of uncoupling protein 3 (UCP3) while reducing mitochondrial ROS production in a regular soccer training period, and also to reduce oxidative damage markers and increase Cu/Zn-SOD protein levels in response to acute exercise [19]. In neutrophils, DHA diet supplementation did not modify the adaptive response of the antioxidant system to training or ROS production induced by immune stimulation after acute exercise while training increased antioxidant defenses (CAT, GPx and GRd enzyme activities) and decreased oxidative damage markers (malonyldialdehyde, carbonyl and nitrotyrosine indexes) [20]. The effects of DHA, training season and acute exercise on the oxidative stress in erythrocytes are not clearly known.
There are several studies analyzing the effects of both DHA and eicosapentaenoic acid (20:5n-3; EPA) (together or individually) and fish oil supplementation on immune cell composition and function during exercise [23, 24]. The aim of the present study was to determine the effects of diet supplementation with DHA on the fatty acid composition of erythrocytes and oxidative balance after an 8-week training season and after acute exercise in soccer players. The influence of training season, acute exercise and DHA supplementation on erythrocyte antioxidant enzyme activities and levels and on the oxidative and nitrosative damage markers was determined.
Materials and Methods
Participants and Study Design
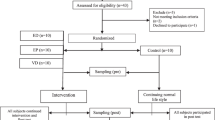
A double blind, randomized supplementation study was performed with 15 male soccer players, at the beginning of their annual sport season. Participants were randomly assigned to be included in two groups: placebo (n = 6) and experimental (n = 9). Participants in the study had a daily intake each morning before the training session of 1 L of their respective beverages for 5 days weekly (excluding the match day and the day of rest), over a total period of 8 weeks (Fig. 1). Inclusion/exclusion criteria were: age (16–35 years), sex (male), non-smokers, balanced diet, body mass index (19–25 kg/m2) and physical activity of 1–2 h daily 5–7 days/week. All the participants were informed of the purpose and demands of the study before giving their written consent to participate. The study protocol was in accordance with the Declaration of Helsinki for research on human participants and was approved by the Ethical Committee of Clinical Investigation of the Comunidad Autónoma de les Illes Balears No IB 994/08 PI (Palma de Mallorca, Balearic Islands, Spain). The project was registered at ClinicalTrials.gov (NCT02177383).
Drink Composition
The two drinks were made up of 3.0 wt% almond with water, lemon (40 mg/L) and cinnamon (200 mg/L) flavors, tocopherol acetate (50 mg/L) and the different oils (olive oil or algal vegetable oil) in functions of placebo and experimental beverage. The placebo was 0.8 wt% refined olive oil and the experimental was 0.6 wt% refined olive oil and 0.2 wt% DHA-S (DSM, Columbia, USA). DHA-S is an algal vegetable oil containing a minimum of 35 % 22:6n-3 fatty acid. The DHA-S ingredients are DHA algal oil (Schizochytrium sp.), high oleic sunflower oil (sunflower lecithin, rosemary extract), tocopherols and ascorbyl palmitate (as antioxidants). The DHA-S fatty acid profile used in the present study was: 14:0 (7.4 %), C16:0 (18.8 %), 18:0 (0.9 %), 18:1n-9 (7.9 %), 18:2n-6 (1.0 %), 20:4n6 (0.6 %), 20:5n-3 (1.4 %), 22:5n-6 (15.3 %), 22:6n-3 (39 %) and others (1.7 %). The two almond drinks were prepared by Liquats Vegetals S.A. (Girona, Spain) and were obtained by: bleaching and grinding almonds with water and then centrifuging the mixture to remove insoluble materials. Sucrose (6.0 wt%), lecithin (0.1 wt%), salt (0.02 wt%), tocopherol acetate (0.02 wt%), natural cinnamon (0.02 wt%), lemon flavors (0.004 wt%), and the respective oil for the experimental (olive oil plus DHA-S) or placebo (olive oil) treatment was added. Finally the beverages were sterilized and packed. The two beverages were identical in taste and appearance. The fatty acid composition and α-Tocopherol content of the beverages is shown in Table 1. 1 L of beverage had 490 kcal. Intake of 1 L of the experimental drink for 5 days a week provided 1.14 g DHA/day.
Experimental Procedure
For each participant, three different blood samples were obtained (Fig. 1). One blood sample was taken under resting conditions at the beginning of the nutritional intervention (week 0, baseline). Another two blood samples were taken at the end of the nutritional intervention, in resting (week 8, pre-exercise) and post-exercise conditions (week 8, post-exercise). 1 L of beverage, placebo or experimental, was consumed before the physical activity session at week 8. The exercise consisted of a 2-h usual physical training session. After a 15 min warm-up, the participants performed the Leger Boucher test [25]. After this, they did a recovery exercise of control-passing over 15 min. The main body of the training session was characterized by small-sided games [19, 20]. The exercise was planned to be performed at 70 % VO2max for more than 50 % of the training session in order to induce oxidative stress [26].
Venous blood samples were obtained from the antecubital vein of participants with two vacutainers containing EDTA (ethylenediaminetetraacetic acid) as anticoagulant for hemogram analyses (2 mL) and to purify erythrocytes (6 mL) following an adaptation of the method described elsewhere [27, 28]. Erythrocyte fraction was obtained after centrifugation (900×g, 30 min, 4 °C). Then, erythrocytes were cleaned with phosphate buffered saline (PBS), centrifuged (900×g, 20 min, 4 °C) and lysed with water at the initial blood volume. Cell lysates were stored at −80 °C until biochemical analyses. Venous blood samples were obtained after 12 h, overnight, fasted conditions (basal sample), and 2 h after finishing training as this is coincident with an increment in circulating immune cells, with changes in antioxidant enzymes activities and in markers of oxidative damage [29, 30].
Hematological Analysis
Hematological parameters such as erythrocyte number, mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH), were determined in an automatic flow-cytometer analyzer Technicon H2 (Bayer, Leverkusen, Germany) VCS system. Hemoglobin concentration was determined using Drabkin reagent to oxidize the heme group leading to the formation of methemoglobin which reacts with potassium cyanide forming cyanmethemoglobin, a stable pigment which can be detected spectrophotometrically at 540 nm.
Anthropometry Measurements
Height was determined using a mobile anthropometer (Kawe 44444, Asperg, Germany) to the nearest millimeter, with the participant’s head placed in the Frankfurt plane. Body weight was determined to the nearest 0.1 kg using a digital scale (Tefal, sc9210, Rumilly, France). Participants were weighed with bare feet and wearing light underwear. Waist and hip circumference were measured to the nearest 0.1 cm, using a non-stretchable measuring tape (KaWe, 43972, France). Triceps, subscapular, biceps, iliac crest, supraspinal, abdominal, thigh, and leg skinfold thickness were measured using a Holtain skinfold calliper (Tanner/Whitehouse, Crosswell, Crymych, UK), and the mean of three measurements was used [31]. Participants were asked to stand erect in a relaxed position with both feet placed together on a flat surface during measurements.
Different anthropometric indexes were calculated using these measurements: body mass index [BMI = mass (kg)/squared height (m)]; waist-hip index [waist circumference (cm)/hip circumference (cm)]; fat free mass (FFM = 100 − BF). Body fat percentage was determined from skinfold thickness according to the Carter-Yuhasz equation [32]. All anthropometric measurements were performed by one observer to avoid inter-observer variation.
Dietary Intake
Dietary habits were assessed using a 7-day dietary record completed at the beginning of the study. A qualified dietician verified and quantified the food records. All food items consumed were transformed into nutrients using a computerized program based on the European and Spanish food composition tables [33, 34].
Fatty Acid Determination
Erythrocyte and beverage (250 µL) lipid content was extracted with chloroform/methanol (2:1, by vol) by a modified method of Folch et al. [35, 36], containing 0.01 % butylated hydroxyanisole (BHA) as antioxidant and 20 µL of n-heptadecanoic acid (15 mM) as the internal standard. The resultant organic phase was evaporated under a nitrogen stream at 55 °C. The dry residue was dissolved in 225 µL of n-hexane and 25 µL of Meth-Prep™ II (Grace Davison Discovery Sciences, Columbia, MD, USA) and the derivatization reagent was added. An aliquot of 1 µL was injected into the gas chromatograph with helium as a mobile phase at 2.17 mL/min flux, measured at 150 °C in head column. The gas chromatograph was an Agilent 5890 model (Agilent Technologies, Santa Clara, CA, USA) with a flame ionization detector (FID) and the column was a Supelcowax® 10 Capillary GC column, 30 m × 0.53 mm, d f 0.50 µm (Supelco, Bellefonte, PA, USA). The temperature curve began at 150 °C with a temperature gradient of 4 °C/min up to 260 °C which was maintained for 15 min. The injector was at 280 °C and the FID at 300 °C. Individual fatty acids and a mix of fatty acid methyl esters (Supelco®) were used for the identification of the chromatography peaks.
Quantification was performed from the internal standard (17:0) and the responses of the fatty acids in the FID was corrected by a response factor calculated from the areas of the standard fatty acids of different chromatograms with different fatty acid concentrations and the area of the internal standard obtained from the same chromatograms. The coefficient of variation was calculated to be 15 %.
α-Tocopherol Determination
α-Tocopherol was determined in the placebo and experimental drinks. The extraction of lipid soluble vitamins was carried out using n-hexane after deproteinization with ethanol containing 0.2 % butylated hydroxytoluene (BHT). α-Tocopherol concentration was determined after drying the samples under nitrogen current and dissolving in methanol. The mobile phase consisted of acetonitrile/tetrahydrofuran/water (550:370:80, by vol.). The HPLC was a Shimadzu (Canby, OR, USA) with a diode array detector and the column was a Nova Pak, C18, 3.9 × 150 mm. α-Tocopherol was determined at 290 nm. Quantification was carried out with an external patron (Sigma-Aldrich).
Malondialdehyde Determination
Malondialdehyde (MDA) as a marker of lipid peroxidation was analyzed in 1/100 diluted erythrocytes using a colorimetric assay kit (Calbiochem). Briefly, samples and standards were placed in 1.5 mL tubes containing n-methyl-2-phenylindole (10.3 mM) in acetonitrile/methanol (3:1, by vol.). HCl 12 N was added and the samples were incubated for 1 h at 45 °C. Absorbance was measured at 586 nm. The method used is specific for MDA determination [37, 38].
Assay of Nitrotyrosine and Protein Carbonyls
Protein carbonyl derivatives and nitrotyrosine (N-Tyr) were determined by immunological methods using the OxiSelect™ Protein Carbonyl Immunoblot Kit (Cell Biolabs, INC) and OxiSelect™ Nitrotyrosine Immunoblot Kit (Cell Biolabs, INC) following the manufacturer’s instructions. Total protein concentrations were measured by the method of Bradford [39]. Initially, erythrocyte samples (10 or 150 µg of protein for carbonyl or N-Tyr, respectively) were transferred onto a nitrocellulose membrane by the dot blot method. For carbonyl determination, the membrane was incubated in the presence of 2,4-dinitrophenylhydrazine (DNPH) after transference. Then the membrane was incubated with the primary antibody, specific to DNP moiety proteins in the case of carbonyl determination, or rabbit anti-N-Tyr antibody for N-Tyr determination. This step was followed by incubation with a horseradish peroxidase-antibody (goat anti-rabbit IgG) conjugate directed against the primary antibody. The membrane was then treated with luminol, which is converted to a light-emitting form at wavelength 428 nm by the antigen/primary antibody/secondary antibody/peroxidase complex. The light was visualized and detected by short exposure to a Chemidoc XRS densitometer (Bio-Rad Laboratories). Image analysis was performed using Quantity One-1D analysis software (Bio-Rad Laboratories). The coefficient of variation has been calculated to be 10 % for carbonyl index and 12 % for N-Tyr index.
Enzymatic Determinations
Erythrocyte catalase (CAT) activity was measured by the spectrophotometric method of Aebi [40]. Results are expressed referring to the constant K of the first order reaction of decomposition of hydrogen peroxide (K = 3.4 × 107 L/mol/s for pure catalase from human erythrocytes) [41]. Superoxide dismutase (SOD) activity was measured in erythrocytes by an adaptation of the method of McCord and Fridovich [42]. Glutathione reductase (GRd) activity was measured in erythrocytes by a modification of the Goldberg and Spooner spectrophotometric method [43]. Glutathione peroxidase (GPx) activity was measured using an adaptation of the spectrophotometric method of Flohé and Gunzler [44]. All activities were determined in erythrocyte samples with a Shimadzu UV-2100 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) at 37 °C.
Western Blot Analysis
Antioxidant enzyme protein levels of erythrocyte samples were determined by Western blot. Protein extracts were analyzed by SDS–polyacrylamide gel electrophoresis (SDS-PAGE). CAT (20 μg of protein), Cu/Zn-SOD (10 μg of protein) and GPx (200 μg of protein) were loaded on a 12 % SDS-PAGE gel, whereas GRd (10 μg of protein) was loaded on a 15 % SDS-PAGE gel. Following electrophoresis, samples were transferred onto a nitrocellulose membrane and incubated with a primary monoclonal antibody: anti-CAT antibody (Calbiochem), anti-Cu/Zn-SOD antibody (Sigma) and anti-GRd antibody and anti-GPx antibody (Santa Cruz). Then incubation with a secondary peroxidase-conjugated antibody was performed. Protein bands were visualized by Immun-Star® Western C® Kit reagent (Bio-Rad Laboratories) Western blotting detection systems. The chemiluminiscence signal was captured with a Chemidoc XRS densitometer (Bio-Rad Laboratories, Hercules, CA, USA) and analyzed with Quantity One-1D Software (Bio-Rad Laboratories).
Statistical Analysis
Statistical analysis was carried out using the Statistical Package for Social Sciences (SPSS v.21.0 for Windows). Results are expressed as means ± SEM. and P < 0.05 was considered statistically significant. The Shapiro–Wilk W test was applied to assess the normal distribution of the experimental data. To test the effects of supplementation and training or supplementation and acute exercise, a two-way analysis of variance (ANOVA) was performed. When significant effects were found, one-way ANOVA was used to determine the differences between the groups.
Results
Twenty-two participants were initially recruited but seven dropped out of the study (six of them left the soccer team and one had an injury) as explained in the consort flow diagram (Fig. 2). Athletes’ nutritional intake before the nutritional intervention was similar in both groups. The reported energy intake was for active people, whose energy and protein intake should be 20 % higher than the general population [45]. Participants’ protein intake was greater than the general recommendations while carbohydrate and fiber intakes were lower [45]. Participant diets had a high quantity of cholesterol and lipids with a poor balance of saturated to unsaturated fats in relation to dietary guidelines, a pattern observed in the general Balearic Island population [46]. Diet supplementation with the placebo and experimental beverages increased PUFA intake. DHA intake by participants was lower than recommendations [15].
The fatty acid composition of the experimental and placebo beverages is shown in Table 1. The experimental drink contained significantly higher concentrations of the fatty acids 20:3 (20:3n-6 and 20:3n-3 fatty acids mixture), 22:0, 22:5 and 22:6n-3, whereas they were undetected in the placebo drink. The experimental beverage also contained significantly higher concentrations of 16:0, 16:1, and 20:1n-9 fatty acids compared to the placebo beverage, when the results are expressed as mg/100 mL. There was no significant difference in α-Tocopherol concentration between the two drinks.
There were no differences in anthropometric characteristics or physical activity capabilities between the placebo and experimental group of athletes at the beginning of the study (Table 2). No differences were observed in nutrient intake except for fiber between the placebo and experimental groups at baseline (Table 3). The intake of polyunsaturated lipids was about 11 g daily in both groups before the nutritional intervention. Diet supplementation with 1 L per day of the beverage provided an increment of about 12 % of the total lipid intake. Experimental group intakes were approximately 1.07, 4.64 and 3.93 g/day of additional saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and PUFA, respectively, whereas the placebo group intake of additional SFA, MUFA and PUFA amounted to 0.57, 2.21 and 1.28 g/day, respectively. DHA consumption from the diet ranged from 68.5 to 150 mg/day according to the 7-day dietary record analysis.
Table 4 illustrates erythrocyte fatty acid composition comparing the baseline (initial) and week 8 pre-exercise (final) conditions. SFA, MUFA, PUFA and total fatty acids in erythrocytes were unchanged by the nutritional intervention in either group. The main effect found was for the erythrocyte content of DHA, which significantly increased in the experimental group, reaching values 1.3 times higher than placebo group. DHA supplementation significantly increased the concentration of the 20:3 mixture of fatty acids, although this value was also different between groups at the beginning of the study. Exercise had little influence on erythrocyte fatty acid composition, irrespective of the placebo or experimental group, comparing the 8-week pre- and post-exercise conditions (Table 5). However, a significant increase in 18:3n-3 fatty acid concentration (about 63 %) after exercise was detected in erythrocytes of the placebo group. Differences in DHA content after the supplementation period were maintained after acute exercise. The 22:0 fatty acid concentration significantly increased only in the experimental group after exercise. The concentration of 18:3n-3 and the 20:3 mixture of fatty acids were significantly lower (about 63 and 34 % respectively) in the experimental group in resting conditions, whereas the concentration of 22:0 and 22:6n-3 fatty acids were about 63 and 39 % significantly higher in the experimental group after exercise. EPA levels in erythrocytes were below detection limits, probably due to the low fish intake of soccer players.
Neither nutritional intervention nor training affected erythrocyte characteristics (Table 6). Erythrocyte counts, hemoglobin, hematocrit, MCV, MCH, MCHC and RDW maintained the basal initial values after 8 weeks of nutritional intervention in both the placebo and experimental groups. Acute exercise and dietary supplementation induced a higher MCHC value after exercise in the experimental group.
Supplementation with DHA did not affect MDA erythrocyte levels (an indicator of lipid peroxidation), carbonyl index (an indicator of protein modification) or N-Tyr index (an indicator of nitrosative damage) (Table 7). However, MDA levels significantly increased 1.5 times in both the placebo and experimental group after the training season. The N-Tyr index significantly decreased at the end of the training season in both groups. Acute exercise and DHA diet supplementation did not affect MDA levels or carbonyl index. The N-Tyr index significantly increased in the placebo group after exercise.
DHA dietary supplementation and training significantly affected the activity and protein levels of antioxidant enzymes in erythrocytes (Table 8a, b). Figure 3 shows a representative blot of different erythrocyte antioxidant protein levels in groups and conditions studied. CAT activity was significantly increased at the end of the training period in both groups. An interaction between DHA supplementation and training was observed in the activity of SOD and GPx. SOD activity was higher in the experimental group at the end of the training season, whereas GPx activity was higher in the placebo group. Protein levels of CAT, Cu/Zn-SOD and GPx were not affected by supplementation or training. GRd activity increased significantly at the end of the study in both groups; whereas its respective protein levels showed higher values in the experimental group at the end of training.
Table 8a, b shows the effects of acute exercise on antioxidant enzyme activities and protein levels at the end of the nutritional intervention. Erythrocytes in the experimental group had lower CAT and GPx activities than the placebo group in resting conditions; however, the basal values of SOD and GR activities were similar in placebo and experimental groups. Acute exercise and diet supplementation significantly changed GPx activity and maintained CAT, SOD and GR basal activities after exercise. GPx activity decreased after exercise in the experimental group. The protein levels of these antioxidant enzymes were not significantly affected by dietary supplementation or acute exercise, with the exception of GRd protein levels. The experimental group had significantly higher erythrocyte GRd protein levels after exercise than the placebo group.
Discussion
Nutritional Intervention
Supplemental intake with the experimental beverage of DHA (1.14 g/day) was lower than that used in nutritional intervention studies with the general population [12, 24] or trained men [13, 14, 18] but was similar to that used in studies with healthy people [11, 47]. The daily intake of the experimental drink increased DHA levels in erythrocyte membranes, in accordance with other studies [11, 12], although no changes were observed in SFA, MUFA, total PUFA and total fatty acids. Changes in the lipid composition of erythrocytes indicated that the participants followed the prescribed beverage intake during the trial and this was effective in incorporating DHA into erythrocytes. The beverages, experimental more than placebo, did present long chain n-3 fatty acids other than DHA; however, none of these other fatty acids were significantly increased in erythrocytes over the supplementation period. The potential contribution of these other long chain fatty acids to the results obtained cannot be ruled out, but at their dosage in the experimental beverages they are unable to alter the fatty acid composition of erythrocytes.
Effects of Exercise on the Fatty Acid Composition of Erythrocytes
Membrane fatty acid composition is a dynamic system and its control and regulation is not clearly understood [48]. Dietary fatty acid intake is known to significantly affect the incorporation of fatty acids into cell membranes, thereby influencing their function [12]. Exercise, regardless of intake, can modify cell fatty acid profiles in different tissue types [49]. An increased fluidity of red blood cell membranes has been observed after chronic exercise [50], and depending on the intensity and sport-type, erythrocyte fatty acid composition may be altered [48, 50]. We found a significant increase in 18:3n-3 in erythrocyte membranes after acute exercise. This is the first time that a rapid change in membrane composition has been observed following exercise in humans. However, several studies performed with rats showed an effect of acute exercise on the fatty acid composition of erythrocytes, which was influenced by aging, training status and body temperature [51, 52].
Effect of Supplementation, Training, and Exercise on Oxidative Stress
EPA and DHA dietary supplementation can modulate erythrocyte membrane deformability and the capacity of O2 transport [53]; similarly, PUFA dietary intake adapts mitochondria to use fatty acids as a fuel and increases their energy efficiency [54], thereby enhancing exercise performance [53, 55, 56]. Oxidative damage is more prominent in red cells, probably due to their high iron and PUFA content, their role as an oxygen transporter, and their protection of the host by neutralizing exogenous and endogenous free radicals [8]. Oxidative damage in the lipid fraction of erythrocytes significantly increased after the dietary intervention in both groups, although no additional change after the acute exercise at week 8 was observed. This increment in oxidative damage occurred over the soccer season with its accumulation of workouts and matches [57]. It has been pointed out that regular exercise in young athletes may be beneficial in reducing the amount of lipid peroxidation and increasing the activity of antioxidant enzymes [58]. The observed antioxidant enzyme activity increases in erythrocytes after training did not prevent oxidative damage of the erythrocyte lipid fraction. In a study performed with handball athletes over 6 months, erythrocyte antioxidant enzymes were significantly increased in a similar magnitude as in the present results. Moreover, the chronic adaptations to training demonstrated a significant protective effect against oxidative stress in erythrocytes, evidenced with decreased thiobarbituric acid reactive substances (TBAR) and carbonyl index [59]. Diet supplementation with DHA did not alter the pattern of oxidative damage in erythrocytes observed in the athletes during or after the training season. The absence of oxidative damage in lipids and proteins after exercise could be a consequence of the training status or the intensity of the training session. Intense exercise such as a mountain cycling or maximal exercise testing has induced oxidative damage in erythrocytes [8, 60], whereas in submaximal exercise, no significant differences have been obtained in MDA levels [60].
Neither training and exercise nor DHA supplementation affected protein oxidative damage measured as carbonyl index. The efficiency of dietary fish oil to reduce in vivo oxidative damage of proteins in rats, may differ due to variations in EPA/DHA content of studies [61]. Protein carbonylation is an important irreversible modification that increases during oxidative stress [62]. ROS formed in exercise initiates PUFA peroxidation to form lipid peroxidation end products such as MDA, which could result in the formation of carbonyl groups on the protein. Therefore, the formation of carbonylated proteins could be unrelated to the formation of MDA. The absence of changes in carbonylated proteins, together with the slight increase in MDA, indicates increased susceptibility to oxidation by lipids; and the MDA produced is too scarce for reaction with the proteins. The results also suggest that the production of ROS was not enough to directly oxidize the proteins in the presence of the erythrocyte antioxidant.
N-Tyr index was similarly decreased in both groups after the training season, although acute exercise increased the N-Tyr index in the placebo group, suggesting that DHA dietary supplementation reduced the nitrosative modifications induced by exercise. Superoxide anion produced in the erythrocytes can react with nitric oxide to form peroxynitrite, which can react with peptide-bound tyrosine to form N-Tyr [63]. In a situation of increased erythrocyte production of nitric oxide after exercise [28], the elimination of superoxide anions is of great importance in order to reduce peroxynitrite and N-Tyr production. SOD eliminates the anion superoxide that is necessary for the formation of peroxynitrite and N-Tyr. The increased SOD observed after activity training in the DHA supplemented group could explain the low N-Tyr index in erythrocytes at the end of the training period, as well as the maintenance of the value after acute exercise.
Erythrocyte antioxidant enzyme activities increased as a result of training, in accordance with preventive erythrocyte antioxidant protection induced by regular exercise [58]. DHA dietary supplementation exerted a differential influence on basal and post-exercise erythrocyte antioxidant enzyme activities. DHA dietary supplementation enhanced the effect of training on erythrocyte SOD activity, but diminished the training effect on GPx and CAT activities. A previous study with long distance skiers revealed a decrease in erythrocyte SOD activities in the blood following an acute bout of exercise [64]. However, it was also reported that no changes were found in erythrocyte SOD activity in trained individuals following a duathlon competition [7]. Antioxidant enzyme activities in erythrocytes are modulated by acute exercise [7] and this modulation is influenced by the presence of low molecular weight antioxidants such as vitamin C [10]. For SOD, GPx and CAT enzymes, the increased enzyme activity did not parallel enzyme protein content, suggesting direct activation of the existing enzymes [65]. These changes in enzymatic capability could be related to the effects of ROS and low molecular weight antioxidants on the enzymatic protein [7, 57]. No evidence for a direct action of DHA on the catalytic activity of antioxidant enzymes was found, but a reduction in CAT, GPx and GRd catalytic activities and an increase in SOD catalytic activity was evidenced in the DHA supplemented group. An important increase in the protein levels of GRd was appreciated as a result of training, mainly in the DHA supplemented group. This could be related to GRd turnover during the maturation process of reticulocytes to erythrocytes, as this maturation has been reported to cause a significant decrease in antioxidant enzyme activities such as GRd, GPx, glutathione S-transferase, glucose-6-phosphate dehydrogenase, and CAT [66]. DHA dietary supplementation may induce higher GRd production in reticulocytes or possibly the GRd of reticulocytes was protected from degradation in the maturation process.
Conclusion
In summary, the consumption of a DHA-enriched almond drink for 8 weeks by professional athletes changed erythrocyte membrane composition without altering lipid oxidative damage markers. The enhanced erythrocyte SOD activity induced by DHA supplementation during the training season paralleled the reduction of peroxidative damage of erythrocyte proteins induced by training or acute exercise. The effects on erythrocyte antioxidant enzymes activities are not attributed to changes in the protein level of antioxidant enzymes but to catalytic capabilities. However, GRd increased its protein levels as a result of the interaction between training and DHA supplementation, pointing to an influence of DHA on GRd turnover during the maturation of reticulocytes to erythrocytes. DHA dietary supplementation promoted greater erythrocyte antioxidant defenses and less protein peroxidative damage in professional athletes during the training season and after acute exercise.
Abbreviations
- ANOVA:
-
Analysis of variance
- BHA:
-
Butylated hydroxyanisole
- BHT:
-
Butylated hydroxytoluene
- CAT:
-
Catalase
- DNPH:
-
2,4-Dinitrophenylhydrazine
- DHA:
-
Docosahexaenoic acid
- EDTA:
-
Ethylenediaminetetraacetic acid
- EPA:
-
Eicosapentaenoic acid
- FID:
-
Flame ionization detector
- GPx:
-
Glutathione peroxidase
- GRd:
-
Glutathione reductase
- MCH:
-
Mean corpuscular hemoglobin
- MCV:
-
Mean corpuscular volume
- MDA:
-
Malondialdehyde
- MUFA:
-
Monounsaturated fatty acid(s)
- PAGE:
-
Polyacrylamide gel electrophoresis
- PBS:
-
Phosphate buffered saline
- PBMC:
-
Peripheral blood mononuclear cell
- PUFA:
-
Polyunsaturated fatty acid(s)
- ROS:
-
Reactive oxygen species
- SFA:
-
Saturated fatty acids
- SOD:
-
Superoxide dismutase
- TBAR:
-
Thiobarbituric acid reactive substance
- UCP:
-
Uncoupling protein
References
Gomez-Cabrera MC, Viña J, Ji LL (2009) Interplay of oxidants and antioxidants during exercise: implications for muscle health. Phys Sportsmed 37:116–123
Gomez-Cabrera MC, Domenech E, Viña J (2008) Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 44:126–131
Fisher-Wellman K, Bloomer RJ (2009) Acute exercise and oxidative stress: a 30 year history. Dyn Med 8:1
Fialkow L, Wang Y, Downey GP (2007) Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med 42:153–164
Halliwell B (2007) Biochemistry of oxidative stress. Biochem Soc Trans 35:1147–1150
Ferrer MD, Sureda A, Mestre A, Tur JA, Pons A (2010) The double edge of reactive oxygen species as damaging and signaling molecules in HL60 cell culture. Cell Physiol Biochem 25:241–252
Tauler P, Gimeno I, Aguiló A, Guix MP, Pons A (1999) Regulation of erythrocyte antioxidant enzyme activities in athletes during competition and short-term recovery. Pflugers Arch 438:782–787
Sureda A, Tauler P, Aguiló A, Cases N, Fuentespina E, Córdova A, Tur JA, Pons A (2005) Relation between oxidative stress markers and antioxidant endogenous defences during exhaustive exercise. Free Radic Res 39:1317–1324
Faivre-Fiorina B, Caron A, Labrude P, Vigneron C (1998) Erythrocyte, plasma and substitute hemoglobins facing physiological oxidizing and reducing agents. Ann Biol Clin (Paris) 56:545–556
Tauler P, Aguiló A, Gimeno I, Fuentespina E, Tur JA, Pons A (2003) Influence of vitamin C diet supplementation on endogenous antioxidant defences during exhaustive exercise. Pflugers Arch 446:658–664
Barceló-Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK (2008) Flaxseed oil and fish-oil capsule consumption alters human red blood cell n-3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n-3 fatty acid. Am J Clin Nutr 88:801–809
Egert S, Lindenmeier M, Harnack K, Krome K, Erbersdobler HF, Wahrburg U, Somoza V (2012) Margarines fortified with α-linolenic acid, eicosapentaenoic acid, or docosahexaenoic acid alter the fatty acid composition of erythrocytes but do not affect the antioxidant status of healthy adults. J Nutr 142:1638–1644
Bloomer RJ, Larson DE, Fisher-Wellman KH, Galpin AJ, Schilling BK (2009) Effect of eicosapentaenoic and docosahexaenoic acid on resting and exercise-induced inflammatory and oxidative stress biomarkers: a randomized, placebo controlled, cross-over study. Lipids Health Dis 8:36
Toft AD, Thorn M, Ostrowski K, Asp S, Moller K, Iversen S, Hermann C, Sondergaard SR, Pedersen BK (2000) N-3 polyunsaturated fatty acids do not affect cytokine response to strenuous exercise. J Appl Physiol 89:2401–2406
Tur JA, Bibiloni MM, Sureda A, Pons A (2012) Dietary sources of omega 3 fatty acids: public health risks and benefits. Br J Nutr 107(Suppl 2):S23–S52
Taccone-Gallucci M, Manca-di-Villahermosa S, Battistini L, Stuffler RG, Tedesco M, Maccarrone M (2006) N-3 PUFAs reduce oxidative stress in ESRD patients on maintenance HD by inhibiting 5-lipoxygenase activity. Kidney Int 69:1450–1454
Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ (2003) Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med 35:772–781
McAnulty SR, Nieman DC, Fox-Rabinovich M, Duran V, McAnulty LS, Henson DA, Jin F, Landram MJ (2010) Effect of n-3 fatty acids and antioxidants on oxidative stress after exercise. Med Sci Sports Exerc 42:1704–1711
Capó X, Martorell M, Sureda A, Llompart I, Tur JA, Pons A (2014) Diet supplementation with DHA-enriched food in football players during training season enhances the mitochondrial antioxidant capabilities in blood mononuclear cells. Eur J Nutr
Martorell M, Capó X, Sureda A, Tur JA, Pons A (2014) Effects of docosahexaenoic acid diet supplementation, training, and acute exercise on oxidative balance in neutrophils. Appl Physiol Nutr Metab 39:446–457
Ferrer MD, Tauler P, Sureda A, Pujol P, Drobnic F, Tur JA, Pons A (2009) A soccer match’s ability to enhance lymphocyte capability to produce ROS and induce oxidative damage. Int J Sport Nutr Exerc Metab 19:243–258
Sureda A, Ferrer MD, Tauler P, Maestre I, Aguiló A, Córdova A, Tur JA, Roche E, Pons A (2007) Intense physical activity enhances neutrophil antioxidant enzyme gene expression. Immunocytochemistry evidence for catalase secretion. Free Radic Res 41:874–883
Kew S, Mesa MD, Tricon S, Buckley R, Minihane AM, Yaqoob P (2004) Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. Am J Clin Nutr 79:674–681
Gray P, Gabriel B, Thies F, Gray SR (2012) Fish oil supplementation augments post-exercise immune function in young males. Brain Behav Immun 26:1265–1272
Léger L, Boucher R (1980) An indirect continuous running multistage field test: the Université de Montréal track test. Can J Appl Sport Sci 5:77–84
Sureda A, Ferrer MD, Tauler P, Romaguera D, Drobnic F, Pujol P, Tur JA, Pons A (2009) Effects of exercise intensity on lymphocyte H2O2 production and antioxidant defences in soccer players. Br J Sports Med 43:186–190
Boyum A (1964) Separation of White Blood Cells. Nature 204:793–794
Sureda A, Batle JM, Tauler P, Ferrer MD, Tur JA, Pons A (2006) Vitamin C supplementation influences the antioxidant response and nitric oxide handling of erythrocytes and lymphocytes to diving apnea. Eur J Clin Nutr 60:838–846
Ferrer MD, Tauler P, Sureda A, Tur JA, Pons A (2009) Antioxidant regulatory mechanisms in neutrophils and lymphocytes after intense exercise. J Sports Sci 27:49–58
Sureda A, Cordova A Ferrer MD, Tauler P, Perez G, Tur JA, Pons A (2009) Effects of l-citrulline oral supplementation on polymorphonuclear neutrophils oxidative burst and nitric oxide production after exercise. Free Radic Res 43:828–835
Ervin RB (2009) Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report:1–7
Carter JELYM (1984) Skinfolds and body composition of Olympic athletes. Med Sport Sci 18:144–182
Tauler P, Ferrer MD, Romaguera D, Sureda A, Aguilo A, Tur J, Pons A (2008) Antioxidant response and oxidative damage induced by a swimming session: influence of gender. J Sports Sci 26:1303–1311
MeM Bibiloni, Pich J, Pons A, Tur JA (2013) Body image and eating patterns among adolescents. BMC Public Health 13:1104
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Lladó I, Palou A, Pons A (1993) Combined enzymic and chromatographic techniques to determine specific radioactivity in free and triglyceride fatty acid plasma fractions. J Chromatogr 619:21–28
Gérard-Monnier D, Erdelmeier I, Régnard K, Moze-Henry N, Yadan JC, Chaudière J (1998) Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem Res Toxicol 11:1176–1183
Erdelmeier I, Gérard-Monnier D, Yadan JC, Chaudière J (1998) Reactions of N-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Mechanistic aspects of the colorimetric assay of lipid peroxidation. Chem Res Toxicol 11:1184–1194
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Bermeyer J (1983) Methods of enzymatic analysis. Enzymes 1: oxidoreductases, transferases, 3rd edn, vol III. Verlag Chemie, Wiley, New York, pp 280
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Goldberg DM SR(1984) Glutathione reductase. In: Methods in enzymatic analysis. pp 258–265 (258–265)
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Ortega RM LA, Requejo AM et al (2004) La composición de los alimentos. Herramienta básica para la valoración nutricional
Tur JA, Romaguera D, Pons A (2005) Does the diet of the Balearic population, a Mediterranean-type diet, ensure compliance with nutritional objectives for the Spanish population? Public Health Nutr 8:275–283
Filaire E, Massart A, Portier H, Rouveix M, Rosado F, Bage AS, Gobert M, Durand D (2010) Effect of 6 Weeks of n-3 fatty-acid supplementation on oxidative stress in Judo athletes. Int J Sport Nutr Exerc Metab 20:496–506
Tepsic J, Vucic V, Arsic A, Blazencic-Mladenovic V, Mazic S, Glibetic M (2009) Plasma and erythrocyte phospholipid fatty acid profile in professional basketball and football players. Eur J Appl Physiol 107:359–365
Helge JW, Ayre KJ, Hulbert AJ, Kiens B, Storlien LH (1999) Regular exercise modulates muscle membrane phospholipid profile in rats. J Nutr 129:1636–1642
Kamada T, Tokuda S, Aozaki S, Otsuji S (1993) Higher levels of erythrocyte membrane fluidity in sprinters and long-distance runners. J Appl Physiol 74:354–358
Teległów A, Dabrowski Z, Marchewka A, Tabarowski Z, Bilski J, Jaśkiewicz J, Gdula-Argasińska J, Głodzik J, Lizak D, Kepińska M (2011) Effects of cold water swimming on blood rheological properties and composition of fatty acids in erythrocyte membranes of untrained older rats. Folia Biol (Krakow) 59:203–209
Teległów A, Bilski J, Dąbrowski Z, Marchewka A, Jaśkiewicz J, Gdula-Argasińska J, Głodzik J, Tabarowski Z, Lizak D (2012) The effects of exercise in water at 4 °C and 25 °C on the rheological properties of blood and the composition of fatty acids in the erythrocyte membranes of laboratory rats. Clin Hemorheol Microcirc 51:139–148
Mickleborough TD (2013) Omega-3 polyunsaturated fatty acids in physical performance optimization. Int J Sport Nutr Exerc Metab 23:83–96
Figueira TR, Barros MH, Camargo AA, Castilho RF, Ferreira JC, Kowaltowski AJ, Sluse FE, Souza-Pinto NC, Vercesi AE (2013) Mitochondria as a source of reactive oxygen and nitrogen species: from molecular mechanisms to human health. Antioxid Redox Signal 18:2029–2074
Huffman DM, Altena TS, Mawhinney TP, Thomas TR (2004) Effect of n-3 fatty acids on free tryptophan and exercise fatigue. Eur J Appl Physiol 92:584–591
Brilla LR, Landerholm TE (1990) Effect of fish oil supplementation and exercise on serum lipids and aerobic fitness. J Sports Med Phys Fitness 30:173–180
Carrera-Quintanar L, Funes L, Viudes E, Tur J, Micol V, Roche E, Pons A (2012) Antioxidant effect of lemon verbena extracts in lymphocytes of university students performing aerobic training program. Scand J Med Sci Sports 22:454–461
Metin G, Atukeren P, Alturfan AA, Gulyasar T, Kaya M, Gumustas MK (2003) Lipid peroxidation, erythrocyte superoxide-dismutase activity and trace metals in young male footballers. Yonsei Med J 44:979–986
Marin DP, Bolin AP, Campoio TR, Guerra BA, Otton R (2013) Oxidative stress and antioxidant status response of handball athletes: implications for sport training monitoring. Int Immunopharmacol 17:462–470
Muñoz Marín D, Olcina G, Timón R, Robles MC, Caballero MJ, Maynar M (2010) Effect of different exercise intensities on oxidative stress markers and antioxidant response in trained cyclists. J Sports Med Phys Fitness 50:93–98
Méndez L, Pazos M, Gallardo JM, Torres JL, Pérez-Jiménez J, Nogués R, Romeu M, Medina I(2012) Reduced protein oxidation in wistar rats supplemented with marine OMEGA-3 pufa. Free Radic Biol Med
Rao RS, Møller IM (2011) Pattern of occurrence and occupancy of carbonylation sites in proteins. Proteomics 11:4166–4173
Pietraforte D, Salzano AM, Marino G, Minetti M (2003) Peroxynitrite-dependent modifications of tyrosine residues in hemoglobin. Formation of tyrosyl radical(s) and 3-nitrotyrosine. Amino Acids 25:341–350
Hubner-Wozniak E, Panczenko-Kresowka B, Lerczak K, Psnik J (1994) Effects of graded treadmill exercise on the activity of blood antioxidant enzymes, lipid peroxides and nonenzymatic anti-oxidants in long-distance skiers. 11:217–226
Hollander J, Bejma J, Ookawara T, Ohno H, Ji LL (2000) Superoxide dismutase gene expression in skeletal muscle: fiber-specific effect of age. Mech Ageing Dev 116:33–45
Sailaja YR, Baskar R, Saralakumari D (2003) The antioxidant status during maturation of reticulocytes to erythrocytes in type 2 diabetics. Free Radic Biol Med 35:133–139
Acknowledgments
Acción Estratégica en Salud del Ministerio de Ciencia e Innovación DPS2008-07033-C03-03, Program of Promotion of Biomedical Research and Health Sciences, Projects 11/01791, Red Predimed-RETIC RD06/0045/1004, CIBERobn CB12/03/30038 and Balearic Island Government (35/2011 and 23/2012) and FEDER funds. We hereby acknowledge the PhD grant provided by the University of the Balearic Islands.
We would like to thank the soccer players involved in the study for their committed participation. The excellent medical assistance of Bartomeu Munar and RCD Mallorca is appreciated.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Martorell, M., Capó, X., Bibiloni, M.M. et al. Docosahexaenoic Acid Supplementation Promotes Erythrocyte Antioxidant Defense and Reduces Protein Nitrosative Damage in Male Athletes. Lipids 50, 131–148 (2015). https://doi.org/10.1007/s11745-014-3976-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-014-3976-6