Abstract
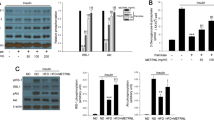
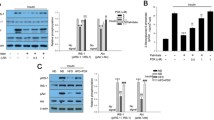
The mammalian target of rapamycin (mTOR) signaling pathway is hyperactive in liver, adipose and skeletal muscle tissues of obese rodents. Alpha-lipoic acid (αLA) has been well accepted as a weight-loss treatment, though there are limited studies on its effect on mTOR signaling in high-fat fed, obese rodents. Therefore, the goal of this study was to determine mTOR signaling and oxidative protein alterations in skeletal muscle of high-fat fed, obese rats after αLA supplementation. Phosphorylation of the mTOR substrate, eukaryotic initiation factor (eIF) 4E-binding protein 1 (4E-BP1) and eIF4B were significantly reduced (p < 0.05) in muscle from αLA supplemented rats. Activation of AMP-activated protein kinase (AMPK), an mTOR inhibitory kinase, was higher (p < 0.05) in the αLA group. Protein expression of markers of oxidative metabolism, acetyl CoA carboxylase (ACC), cytochrome c oxidase IV (COX IV), peroxisome proliferator-activated receptor (PPAR), and PPAR gamma coactivator 1-alpha (PGC-1α) were significantly higher (p < 0.05) after αLA supplementation compared to non-supplemented group. Our findings show that αLA supplementation limits the negative ramifications of consuming a high fat diet on skeletal muscle markers of oxidative metabolism and mTORC1 signaling.




Similar content being viewed by others
Abbreviations
- 4E-BP1:
-
Eukaryotic initiation factor 4E-binding protein 1
- ACC:
-
Acetyl CoA carboxylase
- αLA:
-
Alpha-lipoic acid
- AMPK:
-
AMP-activated protein kinase
- COX IV:
-
Cytochrome c oxidase, subunit IV
- CPT1:
-
Carnitine palmitoyltransferase I
- eIF:
-
Eukaryotic initiation factor
- HF:
-
High fat
- mTOR:
-
Mammalian target of rapamycin
- mTORC1:
-
mTOR complex 1
- PGC-1α:
-
PPAR gamma coactivator 1-alpha
- PPAR:
-
Peroxisome proliferator-activated receptor
- rpS6:
-
Ribosomal protein S6
- S6K1:
-
p70 ribosomal protein S6 kinase-1
- SREBP1c:
-
Sterol regulatory element-binding protein 1c
References
Ogden CL, Carroll MD, Kit BK, Flegal KM (2012) Prevalence of obesity in the United States, 2009–2010. Hyattsville, MD: National Center for Health Statistics. Centers for Disease Control and Prevention’s (CDC) and National Center for Health Statistics (NCHS) NCHS data brief, no 82
Muoio DM, Noland RC, Kovalik JP, Seiler SE, Davies MN et al (2012) Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab 15:764–777
Alley DE, Chang VW (2007) The changing relationship of obesity and disability, 1988–2004. JAMA 298:2020–2027
Srikanthan P, Hevener AL, Karlamangla AS (2010) Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS ONE 5:e10805
Srikanthan P, Karlamangla AS (2011) Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab 96:2898–2903
Durschlag RP, Layman DK (1983) Skeletal muscle growth in lean and obese Zucker rats. Growth 47:282–291
Nilsson MI, Dobson JP, Greene NP, Wiggs MP, Shimkus KL et al (2013) Abnormal protein turnover and anabolic resistance to exercise in sarcopenic obesity. FASEB J 27:3905–3916
Kimball SR, Jefferson LS, Fadden P, Haystead TAJ, Lawrence JC (1996) Insulin and diabetes cause reciprocal changes in the association of eIF4E and PHAS-I rat skeletal muscle. Am J Physiol 270:C705–C709
Kimball SR, Jurasinki CV, Lawrence JC Jr, Jefferson LS (1997) Insulin stimulates protein synthesis in skeletal muscle by enhancing the association of eIF-4E and eIF-4G. Am J Physiol 272:C754–C759
Williamson DL, Li Z, Tuder RM, Feinstein E, Kimball SR et al (2014) Altered nutrient response of mTORC1 as a result of changes in REDD1 expression: effect of obesity vs. REDD1 deficiency. J Appl Physiol (1985) 117:246–256
Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR et al (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110:163–175
Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP et al (2006) Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22:159–168
Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM (1998) RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA 95:1432–1437
Holz MK, Ballif BA, Gygi SP, Blenis J (2005) mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123:569–580
Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D et al (2004) Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J 23:1761–1769
Gingras A-C, Raught B, Sonenberg N (1999) eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. In: Richardson CC (ed) Annual Review of Biochemistry. Annual Reviews, Palo Alto, pp 913–963
Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI et al (2010) Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 39:171–183
Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK et al (2007) mTOR controls mitochondrial oxidative function through a YY1–PGC-1α; transcriptional complex. Nature 450:736–740
Evans RM, Barish GD, Wang Y-X (2004) PPARs and the complex journey to obesity. Nat Med 10:355–361
Kim H, Haluzik M, Asghar Z, Yau D, Joseph JW et al (2003) Peroxisome proliferator-activated receptor-alpha agonist treatment in a transgenic model of type 2 diabetes reverses the lipotoxic state and improves glucose homeostasis. Diabetes 52:1770–1778
Ye JM, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ et al (2001) Peroxisome proliferator-activated receptor (PPAR)-alpha activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-gamma activation. Diabetes 50:411–417
Huss JM, Kopp RP, Kelly DP (2002) Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J Biol Chem 277:40265–40274
Wende AR, Huss JM, Schaeffer PJ, Giguere V, Kelly DP (2005) PGC-1alpha coactivates PDK4 gene expression via the orphan nuclear receptor ERRalpha: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol 25:10684–10694
Morris EM, Jackman MR, Meers GM, Johnson GC, Lopez JL et al (2013) Reduced hepatic mitochondrial respiration following acute high-fat diet is prevented by PGC-1alpha overexpression. Am J Physiol Gastrointest Liver Physiol 305:G868–G880
Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin M-A et al (2008) Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Investig 118:789–800
Hulver MW, Berggren JR, Cortright RN, Dudek RW, Thompson RP et al (2003) Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab 284:E741–E747
Kelley DE, Goodpaster B, Wing RR, Simoneau J-A (1999) Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity and weight loss. Am J Physiol Endocrinol Metab 277:E1130–E1141
Dyck DJ, Peters SJ, Glatz J, Gorski J, Keizer H et al (1997) Functional differences in lipid metabolism in resting skeletal muscle of various fiber types. Am J Physiol 272:E340–E351
Drake JC, Alway SE, Hollander JM, Williamson DL (2010) AICAR treatment for 14 days normalizes obesity-induced dysregulation of TORC1 signaling and translational capacity in fasted skeletal muscle. Am J Physiol Regul Integr Comp Physiol 299:R1546–R1554
Drake JC, Benninger L, Williamson DL (2011) 8-weeks of β-GPA treatment reduces body mass while positively altering translation initiation in obese skeletal muscle. J Obes Weight Loss Ther 1:1–7
Packer L, Witt EH, Tritschler HJ (1995) Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med 19:227–250
Rosenberg HR, Culik R (1959) The effect of alpha-lipoic acid on vitamin C and vitamin E deficiency. Arch Biochem Biophys 80
Kim M-S, Park J-Y, Namkoong C, Jang P-G, Ryu J-W et al (2004) Anti-obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med 10:727–733
Lee WJ, Song K-H, Koh EH, Won JC, Kim HS et al (2005) α-Lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun 332:885–891
Koh EH, Lee WJ, Lee SA, Kim EH, Cho EH et al (2011) Effects of alpha-lipoic acid on body weight in obese subjects. Am J Med 124:85-e81
Carrier B, Wen S, Zigouras S, Browne RW, Li Z et al (2014) Alpha-lipoic acid reduces LDL-particle number and PCSK9 concentrations in high-fat fed obese Zucker rats. PLoS One 9:e90863
Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M et al (2004) Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431:200–205
Khamzina L, Veilleux A, Bergeron S, Marette A (2005) Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology 146:1473–1481
Timmers S, de Vogel-van den Bosch J, Towler MC, Schaart G, Moonen-Kornips E et al (2010) Prevention of high-fat diet-induced muscular lipid accumulation in rats by α lipoic acid is not mediated by AMPK activation. J Lipid Res 51:352–359
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149:274–293
Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18:1926–1945
Williamson DL (2011) Normalizing a hyperactive mTOR initiates muscle growth during obesity. Aging 3:83–84
Takano A, Usui I, Haruta T, Kawahara J, Uno T et al (2001) Mammalian target of rapamycin pathway regulates insulin signaling via subcellular redistribution of insulin receptor substrate 1 and integrates nutritional signals and metabolic signals of insulin. Mol Cell Biol 21:5050–5062
Wang Y, Li X, Guo Y, Chan L, Guan X (2010) alpha-Lipoic acid increases energy expenditure by enhancing adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling in the skeletal muscle of aged mice. Metab, Clin Exp 59:967–976
Dufner A, Thomas G (1999) Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res 253:100–109
Huong DT, Ide T (2008) Dietary lipoic acid-dependent changes in the activity and mRNA levels of hepatic lipogenic enzymes in rats. Br J Nutr 100:79–87
Ricoult SJH, Manning BD (2012) The multifaceted role of mTORC1 in the control of lipid metabolism. EMBO Rep 14:242–251
Yecies JL, Zhang HH, Menon S, Liu S, Yecies D et al (2011) Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab 14:21–32
Li Y, Xu S, Mihaylova MM, Zheng B, Hou X et al (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13:376–388
Park K-G, Min A-K, Koh EH, Kim HS, Kim M-O et al (2008) Alpha-lipoic acid decreases hepatic lipogenesis through adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways. Hepatology 48:1477–1486
Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ et al (2006) AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPAR alpha and PGC-1. Biochem Biophys Res Commun 340:291–295
Wang Y-X, Zhang C-L, Yu RT, Cho HK, Nelson MC et al (2004) Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol 2:e294
Muoio DM, MacLean PS, Lang DB, Li S, Houmard JA et al (2002) Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) alpha knock-out mice. Evidence for compensatory regulation by PPAR delta. J Biol Chem 277:26089–26097
Benton CR, Holloway GP, Han XX, Yoshida Y, Snook LA, et al (2010) Increased levels of peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PGC-1alpha) improve lipid utilisation, insulin signalling and glucose transport in skeletal muscle of lean and insulin-resistant obese Zucker rats. Diabetologia 53:2008–2019
Ruderman NB, Saha AK, Vavvas D, Heydrick SJ, Kurowski TG (1997) Lipid abnormalities in muscle of insulin-resistant rodents––the malonyl CoA hypothesis. Lipids Syndr Insulin Resist: Mol Biol Clin Med 827:221–230
Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB et al (2008) Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab 8:411–424
Acknowledgments
The authors would like to thank Amy Raslawsky for technical support and Dr. John Wilson for laboratory equipment use. The authors would like to thank Nutritional Fundamentals for Health for graciously donating the R+enantiomer alpha-lipoic acid (αLA) used in this study.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Li, Z., Dungan, C.M., Carrier, B. et al. Alpha-Lipoic Acid Supplementation Reduces mTORC1 Signaling in Skeletal Muscle from High Fat Fed, Obese Zucker Rats. Lipids 49, 1193–1201 (2014). https://doi.org/10.1007/s11745-014-3964-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-014-3964-x