Abstract
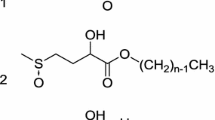
2-Hydroxy-4-(methylthio)butanoic acid (HMTBA) is a commonly used animal feed additive available in large quantities. In this study, anionic surfactants were synthesized utilizing HMTBA as a starting material. Specifically, a straight-chain fatty acid containing 12 or 16 carbon atoms was attached to the hydroxyl group via esterification. After neutralization of the carboxylic acid with sodium, the molecules behave as anionic surfactants. Oxidation of the sulfur atom can be performed to further increase water solubility. The molecules exhibit critical micelle concentrations (CMCs), and lower the surface tension to 35–45 mN/m at the CMC. The derivatives have low Krafft points (<4 °C) and good wetting performance. The hardness tolerance of the ester made from dodecanoic acid is ~2.5–4 orders of magnitude higher than an analogous carboxylate surfactant, namely sodium dodecanoate. Foam created according to the Ross-Miles foam test is substantial, but dissipates quickly as compared to other anionic surfactants.





Similar content being viewed by others
Abbreviations
- HMTBA:
-
2-Hydroxy-4-(methylthio)butanoic acid
- AES:
-
Alkyl ether sulfate
- SDS:
-
Sodium dodecyl sulfate
- SDBS:
-
Sodium dodecylbenzene sulfonate
- CMC:
-
Critical micelle concentration
- γ CMC :
-
Surface tension at the CMC
- Γm :
-
Maximum surface excess concentration/maximum adsorption density
- C20:
-
Surfactant concentration that lowers the surface tension by 20 mN/m
- a min :
-
Minimum area per molecule at the interface
References
Tsoler U (1999) Handbook of detergents. M. Dekker, New York
Smulders E, von Rybinski W, Sung E, Rähse W, Steber J, Wiebel F, Nordskog A (2000) Laundry detergents. Wiley, Germany
Atwood JL, Steed JW (2004) Encyclopedia of supramolecular chemistry. Marcel Dekker, New York
El-Sukkary MMA, Soliman EA, Ismail DA, El Rayes SM, Saad MA (2011) Synthesis and properties of some N-acylethylenediamine triacetic acid chelating surfactants. Tenside Surfactants Deterg 48:82–86
Fujiwara M, Miyake M, Abe Y (1993) Colloidal properties of alpha-sulfonated fatty acid methyl esters and their applicability in hard water. Colloid Polym Sci 271:780–785
Hong J-J, Yang S-M, Choi Y-K, Lee C-H (1995) Precipitation of tricarboxylic acid biosurfactant derived from spiculisporic acid with metal ions in aqueous solution. J Colloid Interface Sci 173:92–103
Laurent JCTRBS, Connor DS, Cripe TA, Dupont JS, Scheibel JJ, Stidham RE, Vinson PK, Willman KW (2000) Mid-chain branched surfactants. US Patent 6,020,303
Laurent JCTRBS, Connor DS, Cripe TA, Dupont JS, Vinson PK, Willman KW (2000) Mid-chain branched alkyl sulfate surfactants. US patent 6,060,443
Laurent JCTRBS, Connor DS, Cripe TA, Vinson PK, Willman KW (1999) Mid-chain branched alkoxylated sulfate surfactants. US Patent 6,008,181
Scheibel J (2004) The evolution of anionic surfactant technology to meet the requirements of the laundry detergent industry. J Surfactants Deterg 7:319–328
Shi J, Kloepper-Sams P, Giolando S, Federle T, Versteeg D, Belanger S (2000) Biodegradable high solubility alkyl sulfate surfactants: environmental safety profiles, in 5th World Surfactant Congress. Federchimica, Milan, pp 1525–1531
Stirton AJ, Bistline RG, Maurer EW, Weil JK, Ault WC (1962) Sodium salts of alkyl esters of alpha-sulfo fatty acids–wetting, lime soap dispersion, and related properties. J Am Oil Chem Soc 39:128–139
van Duynhoven J, Leika A, van der Hoeven R (2005) Quantitative assessment of alkyl chain branching in alcohol-based surfactants by nuclear magnetic resonance. J Surfactants Deterg 8:73–82
Xing F, Niu J, Liu X, Wang X (2014) Effect of a spacer group on surface activity, salinity and hardness tolerance, mimic oil washing efficiency of monododecyl diaryl disulfonate. J Surfactants Deterg 17:95–100
Yu D, Wang Y, Zhang J, Tian M, Han Y, Wang Y (2012) Effects of calcium ions on solubility and aggregation behavior of an anionic sulfonate gemini surfactant in aqueous solutions. J Colloid Interface Sci 381:83–88
Dawe B, Oswald T (1991) Reduced adsorption and separation of blended surfactants on sand and clay. J Can Pet Technol 30:133–137
Lad K, Bahadur A, Pandya K, Bahadur P (1995) Clouding and aggregation behavior of ethylene-oxide propylene-oxide ethylene-oxide block-copolymers in aqueous-media in the presence of sodium dodecyl-sulfate. Indian J Chem Sec a-Inorg Bio-Inorg Physical Theor Anal Chem 34:938–945
Rodriguez CH, Scamehorn JF (1999) Modification of Krafft temperature or solubility of surfactants using surfactant mixtures. J Surfactants Deterg 2:17–28
Sharma R, Desai A, Bahadur P (2003) Hardness tolerance of anionic surfactants in the presence of nonionic surfactants. Tenside Surfactants Deterg 40:31–34
Satsuki T, Nagoh Y, Yoshimura H (1998) Effect of calcium ions on detergency—part 2: interactions between a surfactant, a calcium-sequestering builder and calcium ions. Tenside Surfactants Deterg 35:112–118
Yu YX, Zhao J, Bayly AE (2008) Development of surfactants and builders in detergent formulations. Chin J Chem Eng 16:517–527
Tanthakit P, Nakrachata-Amorn A, Scamehorn JF, Sabatini DA, Tongcumpou C, Chavadej S (2009) Microemulsion formation and detergency with oily soil: V. Effects of water hardness and builder. J Surfactants Deterg 12:173–183
Sweeney W, Anderson R (1989) Biodegradability of alkylbenzene sulfonates. J Am Oil Chem Soc 66:1844–1849
Anonymous (1965) Testing for surfactant biodegradability industrial and engineering chemistry 57: 45–6
Dibner JJ, Knight CD (1984) Conversion of 2-hydroxy-4-(methylthio)butanoic acid to l-methionine in the chick—a stereospecific pathway. J Nutr 114:1716–1723
Schott H, Han SK (1976) Effect of inorganic additives on solutions of nonionic surfactants 4: Krafft points. J Pharm Sci 65:979–981
International ASTM (2007) Standard test method for foaming properties of surface-active agents. West Conshohocken, Pennsylvania
International ASTM (2010) Standard test method for evaluation of wetting agents by the Skein test. West Conshohocken, Pennsylvania
Guzman A, Bueno A, Carbognani L (2009) Molecular weight determination of asphaltenes from Colombian crudes by size exclusion chromatography (SEC) and vapor pressure osmometry (VPO). Pet Sci Technol 27:801–816
Dahanayake M, Cohen AW, Rosen MJ (1986) Relationship of structure to properties of surfactants. 13. Surface and thermodynamic properties of some oxyethylenated sulfates and sulfonates. J Phys Chem 90:2413–2418
Glukhareva NA, Pletnev MY (1995) Krafft points of some mixtures based on individual sodium soaps. Colloid J 57:253–255
Elworthy PH, Mysels KJ (1966) Surface tension of sodium dodecylsulfate solutions and phase separation model of micelle formation. J Colloid Interface Sci 21:331–347
Acevedo S, Gutierrez LB, Negrin G, Pereira JC, Mendez B, Delolme F, Dessalces G, Broseta D (2005) Molecular weight of petroleum asphaltenes: a comparison between mass spectrometry and vapor pressure osmometry. Energy Fuels 19:1548–1560
van Voorst Vader F (1960) Adsorption of detergents at the liquid-liquid interface. Part 1. Trans Faraday Soc 56:1067–1077
DeLisi R, Inglese A, Milioto S, Pellerito A (1997) Demixing of mixed micelles Thermodynamics of sodium perfluorooctanoate sodium dodecanoate mixtures in water. Langmuir 13:192–202
Ingram T, Jones MN (1969) Membrane Potential Studies on Surfactant Solutions. Trans Faraday Soc 65:297–304
Akhter MS (1997) Effect of acetamide on the critical micelle concentration of aqueous solutions of some surfactants. Colloids Surf A Physicochem Eng Asp 121:103–109
Kralchevsky PA, Danov KD, Pishmanova CI, Kralchevska SD, Christov NC, Ananthapadmanabhan KP, Lips A (2007) Effect of the precipitation of neutral-soap, acid-soap, and alkanoic acid crystallites on the bulk pH and surface tension of soap solutions. Langmuir 23:3538–3553
Jackson LP, Townsend C, Grady BP (2013) Mixtures of nonionic surfactants made from renewable resources with alkyl sulfates and sodium n-alkanecarboxylates: comparison of mixing behavior using Rubingh’s treatment. J Surfactants Deterg 16:893–902
Wen X, Franses EI (2000) Effect of protonation on the solution and phase behavior of aqueous sodium myristate. J Colloid Interface Sci 231:42–51
Campbell AN, Lakshmin GR (1965) Conductances and surface tensions of aqueous solutions of sodium decanoate sodium laurate and sodium myristate at 25° and 35°. Can J Chem 43:1729–1737
Tanaka S, Kawasaki H, Maeda H (2005) Complex formation in alkyldimethylamine oxide/sodium palmitate/water mixtures. J Colloid Interface Sci 283:238–244
Blanco E, González-Pérez A, Ruso JM, Pedrido R, Prieto G, Sarmiento F (2005) A comparative study of the physicochemical properties of perfluorinated and hydrogenated amphiphiles. J Colloid Interface Sci 288:247–260
Weil JK, Smith FD, Bistline RG, Stirton AJ (1963) Long chain alkanesulfonates and 1-hydroxy-2-alkanesulfonates—structure and property relations. J Am Oil Chem Soc 40:538–541
Stellner KL, Scamehorn JF (1989) Hardness tolerance of anionic surfactant solutions. 1. Anionic surfactant with added mono-valent electrolyte. Langmuir 5:70–77
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Yu, G., Long, S.A., Karinshak, K.A. et al. Synthesis and Characterization of Novel Surfactants Based on 2-Hydroxy-4-(Methylthio)Butanoic Acid: 1. Anionic Surfactants. J Surfact Deterg 18, 895–903 (2015). https://doi.org/10.1007/s11743-015-1690-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1690-x