Abstract
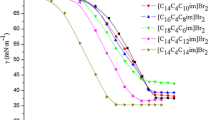
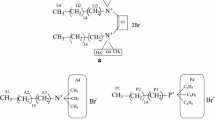
Construction of gemini-like surfactants using the cationic single-chain surfactant cetyltrimethylammonium bromide C16H33N(CH3)3Br2 (CTAB) and the anionic dicarboxylic acid sodium salt NaOOC(CH2) n-2COONa (C n Na2, n = 4, 6, 8, 10, 12) by way of non-covalent interactions has been investigated by surface tension measurements, hydrogen-1 nuclear magnetic resonance (1H NMR) spectroscopy and isothermal titration microcalorimetry (ITC). The critical micelle concentrations (cmc) of the CTAB/C n Na2 mixtures are obviously lower than that of CTAB and strongly depend on the mixing ratio. Moreover, the cmc values of the CTAB/C n Na2 mixtures decrease gradually with an increasing methylene chain length of C n Na2, indicating hydrophobic interaction between the hydrocarbon chains of CTAB and C n Na2 facilitates micellization of the mixtures. In particular, the ITC curves and 1H NMR spectra indicate that the binding ratio of CTAB to C n Na2, except C4Na2, is around 2:1, i.e., (CTAB)2C n Na2. Additionally, CTAB/C n Na2 mixtures are soluble in a whole molar ratio and concentration ranges have been studied, even at the electrical neutralization point. Therefore, these results reveal that highly soluble gemini-like surfactants are conveniently constructed with oppositely-charged cationic single-chain surfactants and dicarboxylic acid sodiums. In an attempt at improving the performance of surfactants this work provides guidance for choosing additives that form gemini-like surfactants via an uncomplicated synthesis.




Similar content being viewed by others
References
Menger FM, Littau CA (1991) Gemini surfactants: synthesis and properties. J Am Chem Soc 113:1451–1452
Menger FM, Keiper JS (2000) Gemini surfactants. Angew Chem Int Ed 39:1906–1920
Zana R (2002) Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: a review. Adv Colloid Interface Sci 97:205–253
Zana R (2002) Dimeric (gemini) surfactants: effect of the spacer group on the association behavior in aqueous solution. J Colloid Interface Sci 248:203–220
Oda R, Huc I, Schmutz M, Candau SJ, MacKintosh FC (1999) Tuning bilayer twist using chiral counterions. Nature 399:566–569
Hait SK, Moulik SP (2002) Gemini surfactants: a distinct class of self-assembling molecules. Curr Sci 82:1101–1111
Song LD, Rosen MJ (1996) Surface properties, micellization, and premicellar aggregation of gemini surfactants with rigid and flexible spacers. Langmuir 12:1149–1153
Han YC, Wang YL (2011) Aggregation behavior of gemini surfactants and their Interaction with macromolecules in aqueous solution. Phys Chem Chem Phys 13:1939–1956
Zana R, Xia JD (2004) Gemini surfactants: synthesis, interfacial and solution-phase behavior, and applications. CRC Press, Boca Raton
Mitjans M, Martínez V, Clapés P, Pérez L, Infante MR, Vinardell MP (2003) Low potential ocular irritation of arginine-based gemini surfactants and their mixtures with nonionic and zwitterionic surfactants. Pharm Res 20:1697–1701
Páhi AB, Király Z, Mastalir Á, Dudás J, Puskás S, Vágó Á (2008) Thermodynamics of micelle formation of the counterion coupled gemini surfactant bis(4-(2-dodecyl) benzenesulfonate)-jeffamine salt and its dynamic adsorption on sandstone. J Phys Chem B 112:15320–15326
Borde C, Nardello V, Wattebled L, Laschewsky A, Aubry JM (2008) A gemini amphiphilic phase transfer catalyst for dark singlet oxygenation. J Phys Org Chem 21:652–658
Bombelli C, Caracciolo G, Di Profio P, Diociaiuti M, Luciani P, Mancini G, Mazzuca C, Marra M, Molinari A, Monti D, Toccacieli L, Venanzi M (2005) Inclusion of a photosensitizer in liposomes formed by DMPC/gemini surfactant: correlation between physicochemical and biological features of the complexes. J Med Chem 48:4882–4891
Caillier L, Taffin de Givenchy E, Levy R, Vandenberghe Y, Geribaldi S, Guittard F (2009) Polymerizable semi-fluorinated gemini surfactants designed for antimicrobial materials. J Colloid Interface Sci 332:201–207
Kirby AJ, Camilleri P, Engberts JB, Feiters MC, Nolte RJ, Söderman O, Bergsma M, Bell PC, Fielden ML, Rodríguez CLG, Guédat P, Kremer A, McGregor C, Perrin C, Ronsin G, van Eijk MCP (2003) Gemini surfactants: new synthetic vectors for gene transfection. Angew Chem Int Ed 42:1448–1457
Paria S (2008) Surfactant-enhanced remediation of organic contaminated soil and water. Adv Colloid Interface Sci 138:24–58
Zhang X, Wang C (2011) Supramolecular amphiphiles. Chem Soc Rev 40:94–101
Wang C, Wang ZQ, Zhang X (2012) Amphiphilic building blocks for self-assembly: from amphiphiles to supra-amphiphiles. Acc Chem Res 45:608–618
Wang C, Wang ZQ, Zhang X (2011) Superamphiphiles as building blocks for supramolecular engineering: towards functional materials and surfaces. Small 7:1379–1383
Sakai H, Okabel Y, Tsuchiya K, Sakai K, Abe M (2011) Catanionic mixtures forming gemini-like amphiphiles. J Oleo Sci 60:549–555
Yu DF, Tian MZ, Fan YX, Ji G, Wang YL (2012) Aggregate transitions in aqueous solutions of sodium dodecylsulfate with a “gemini-type” organic salt. J Phys Chem B 116:6425–6430
Zhu LY, Han YC, Tian MZ, Wang YL (2013) Complex formation and aggregate transitions of sodium dodecyl sulfate with an oligomeric connecting molecule in aqueous solution. Langmuir 29:12084–12092
Wang MN, Fan YX, Han YC, Nie ZX, Wang YL (2013) Coacervation of cationic gemini surfactant with N-Benzoylglutamic acid in aqueous solution. Langmuir 29:14839–14847
Zhang YM, Feng YJ, Wang YJ, Li XL (2013) CO2-switchable viscoelastic fluids based on a pseudogemini surfactant. Langmuir 29:4187–4192
Skold RO, Tunius MAR (1992) Self-association of 1,10-decanedicarboxylates in aqueous solution. J Colloid Interface Sci 152:183–196
Han F, Huang JB, Zheng B, Li ZC (2004) Surface properties of bolaamphiphiles in ethanol/water mixed solutions. Colloids Surf A 242:115–122
Danino D, Talmon Y, Zana R (1995) Alkanediyl-α, ω-bis(dimethylalkyl ammonium bromide) surfactants (dimeric surfactants). 5. Aggregation and microstructure in aqueous solutions. Langmuir 11:1448–1456
Israelachvili JN, Mitchell DJ, Ninham BW (1976) Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J Chem Soc, Faraday Trans 2(72):1525–1568
Wang G, Olofsson G (1998) Titration calorimetric study of the interaction between ionic surfactants and uncharged polymers in aqueous solution. J Phys Chem B 102:9276–9283
Dai S, Tam KC, Li L (2001) Isothermal titration calorimetric studies on interactions of ionic surfactant and poly(oxypropylene)-poly(oxyethylene)-poly(oxypropylene) triblock copolymers in aqueous solutions. Macromolecules 34:7049–7055
Dai S, Tam KC (2004) Isothermal titration calorimetric studies on the temperature dependence of binding interactions between poly(propylene glycol)s and sodium dodecyl sulfate. Langmuir 20:2177–2183
Lapitsky Y, Parikh M, Kaler EW (2007) Calorimetric determination of surfactant/polyelectrolyte binding isotherms. J Phys Chem B 111:8379–8387
Han YC, Xia L, Zhu LY, Zhang SS, Li ZB, Wang YL (2012) Association behaviors of dodecyltrimethylammonium bromide with double hydrophilic block co-polymer poly(ethylene glycol)-block-poly(glutamate sodium). Langmuir 28:15134–15140
Hong L, Bush WD, Hatcher LQ, Simon J (2008) Determining thermodynamic parameters from isothermal calorimetric isotherms of the binding of macromolecules to metal cations originally chelated by a weak ligand. J Phys Chem B 112:604–611
Olofsson G, Loh W (2009) On the use of titration calorimetry to study the association of surfactants in aqueous solutions. J Braz Chem Soc 20:577–593
Acknowledgments
We are grateful for financial supports from Chinese Academy of Sciences and National Natural Science Foundation of China (Grants 21025313, 21321063).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tang, Y., Wang, R. & Wang, Y. Constructing Gemini-Like Surfactants with Single-Chain Surfactant and Dicarboxylic Acid Sodium Salts. J Surfact Deterg 18, 25–31 (2015). https://doi.org/10.1007/s11743-014-1632-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-014-1632-z