Abstract
The aqueous solution behavior of a PEO–PPO–PEO block copolymer (EO103PO39EO103), was investigated in the presence of aliphatic alkanols (C2, C4, C6 and C8). The non-associated polymer chains remain extremely hydrated in water, but aggregation in the form of spherical micelles was evidenced, triggered by the interaction of polymer chains with hydrophobic alkanol. We assume that the hydrophobic interaction between the PPO block of the copolymer and alkanol promotes micellization, which increases further with the introduction of higher chain length species. The critical micellization temperature (CMT), as measured by UV–visible spectroscopy, indicates an interaction of polymer chains with the alkanol bearing a higher chain length, which triggers aggregation. The micelles were characterized by small angle neutron scattering to elucidate the size and related micellar parameters. The gradual increase in the alkanol content increases the aggregation number, though the micelles were spherical in shape. We conclude that ethanol, due to its preferential solubility in the aqueous phase, does not affect the aggregation. The alkanols with chain lengths of C4–C8 chain, interact with the PPO block through hydrophobic interaction and shifts the CMTs to lower values. The combined effect of inorganic salt (NaCl) and alkanols show enhanced micellar properties.
Similar content being viewed by others
Introduction
The ABA type symmetrical tri-block copolymers consisting of poly(propylene oxide) (PPO) as a hydrophobic middle block sandwiched between two hydrophilic blocks of poly(ethylene oxide) (PEO), are commercially available [1]. These PEO–PPO–PEO triblock copolymers constitute an important group of nonionic surfactants used as anti-foams, emulsifiers, wetting agents, solubilizing agents etc. As a result of their reversible thermorheolgical nature and low toxicity they have been used in pharmaceutical formulations especially in the solubilization of hydrophobic drugs, as surface modifiers of nanospheres and liposomes for producing potential drug delivery/targeting systems [2–6]. These aggregates in water and form micelles with a core of PPO surrounded by a hydrated PEO shell. The micellization of these copolymers differs from the conventional nonionic EO based surfactants due to the central PPO block, which loses hydrophobicity at temperatures below 15 °C. The micellization is strongly temperature dependent and several of these copolymers (particularly with low mol wt PPO and very high % PEO) do not micellize at ambient temperatures even at fairly high concentrations (>5 wt% and even more) [3, 4]. Aromatic solubilizates are better solvents for the PPO blocks than aliphatics, and consequently the solubilization capacities for the aromatic molecules were significant, whereas those of the aliphatics were negligible [7]. This effect was confirmed when a mixture of aromatic and aliphatic solubilizates was investigated [8, 9]. Collett and Tobin showed that an optimal PEO/PPO ratio was necessary to maximize the acetanilide solubilization [10]. Most studies deal with solubilization of aromatic and aliphatic hydrocarbons and the literature concerning the solubilization of polar liquids is still rare [11]. Generally, water is used as the solvent for PEO–PPO–PEO block copolymers and is a controllable factor governing the critical micelle concentration (CMC), critical micellization temperature (CMT) and structure of the micelles [12, 13]. The interaction of polar cosolvents presents an extra degree of freedom in tailoring the solution properties for specific applications, for example, in the formulation of aqueous preparations of water-insoluble drugs and cosmetics and as templates in the synthesis of mesoporous materials [14, 15]. The solvent quality plays decisive role in micellization, where addition of hydrophobic solutes can play a major task by promoting hydrophobicity. In our earlier report [16] the micellar behavior of (EO)20(PO)70(EO)20 (CMT <20 °C for 2 % solution) in the presence of alkanols (C1–C6) is discussed, where micellar transition from sphere to rod like micelles was observed for alkanols having higher carbon chain length. In this regards, we found it interesting to study the interaction of alkanols with (EO103PO39EO103), which does not micellize at the ambient temperature. The introduction of alkanols having different solubility character and the accordingly evolved possibility to trigger micellization, prompted us to investigate this idea. The results are discussed using spectral and scattering techniques, which showed good correlation with each other. To the best of our knowledge, a systematic investigation on hydrophilic PEO–PPO–PEO block copolymer aqueous solutions in the presence of series of hydroxyl compounds is seldom attempted. The information can add to the already existing knowledge of the PEO–PPO–PEO block copolymers and their micellization in the presence of polar additives.
Materials and Methods
Pluronic® F88 (EO)103(PO)39(EO)103 was obtained from the BASF Corp., Parsippanny, New Jersey. It will be called F88 in what follows. Its structural formula is given in Fig. 1. Its molecular characteristics are listed in Table 1. Alkanols viz. ethanol, 1-butanol, 1-pentanol, 1-hexanol and 1-octanol employed in this study were of analytical reagent grade. Sodium chloride (NaCl) was purchased from Fluka. Triple distilled water was used to prepare aqueous solutions. D2O was used for preparing solutions for small angle neutron scattering (SANS) measurements.
Cloud Point (CP)
The cloud point was measured by visually observing the temperature at which the copolymer solution becomes turbid on heating and clear on cooling. Heating and cooling rates of 1 °C per minute near the cloud point were maintained. The process was repeated several times and the average temperature was taken as the CP. The results were reproducible within ±0.5 °C.
Fourier Transform IR Spectroscopy (FTIR)
The FTIR spectra were recorded on a Bruker Vector 22 FTIR spectrometer. The temperature of the sample was measured by a thermocouple inserted into a stainless steel block containing the sample cell. The equilibration time for each measurement was 1 min. FTIR spectra of all samples were recorded from 20 to 50 °C by scanning 64 times.
Fluorescence Spectroscopy
The micropolarity in aqueous copolymer solutions was probed as a function of temperature using the intensity of the pyrene vibronic fine structure in fluorescence emission spectra. The fluorescence emission spectra of excited state monomeric pyrene solubilized in the investigated solutions were recorded with a Jasco FP-6500 spectrofluorometer within the range 350–500 nm using an excitation wavelength of 330 nm and a slit width of 15 nm. The fluorescence intensity ratio of the first and third vibronic bands (I 1/I 3) was used as a spectroscopic tool to qualitatively measure the polarity of the probe microenvironment in micelles/unimers.
Differential Scanning Calorimetry (DSC)
DSC measurements were performed with a Microcal MC-2 instrument (Microcal, Amherst, MA, USA). The sample solution was then injected into the sample cell and the reference cell filled with water. Temperature scans were performed at a rate of 1 °C/min and in the range of 10–90 °C.
UV–Visible Spectroscopy
Shimadzu (UV-2450) UV–visible double beam spectrophotometer was used to determine the critical micellization temperature (CMT). The required volume of alkanols was dissolved in polymer solutions with constant stirring. The CMTs of copolymers was determined by iodine UV spectroscopy method [17, 18]. The temperature was scanned at a heating rate of 1 °C/min. The CMT values correspond to the temperature at which the sharp increase in absorbance is observed.
Small Angle Neutron Scattering (SANS)
The SANS experiments were performed using a SANS diffractometer at the Dhruva reactor, BARC, Trombay, India [19]. The solutions were held in a 0.5 cm thick quartz cell with Teflon stoppers. The diffractometer uses a polycrystalline BeO filter as a monochromator. The mean wavelength of the incident neutron beam is 5.2 Å with a wavelength resolution of approximately 15 %. The angular distribution of the scattered neutron was recorded using a linear 1 m-long He3 position sensitive detector (PSD). The scattering wave vector (Q) is defined as,
where, θ = scattering angle and λ = wavelength of incident neutrons. The scattering data were recorded in the Q range of 0.017–0.35 (Å−1). All the measured SANS distributions were corrected for the background and solvent contributions. The data were normalized to the cross-sectional unit using standard procedures. The scattering cross section per unit volume measured as a function of scattering wave vector gives micellar parameters for the monodispersed Pluronic® system [20].
Results and Discussion
Figure 2a shows the FTIR and fluorescence profile for 5 wt% aqueous solution of F88. The FTIR plot depicts observed changes in wave number shift for C–O–C stretching vibration band as a function of temperature. The higher PEO molecular weight contribution of about 80 % for F88, keeps the polymer chains as molecularly dissolved in water, even at fairly high copolymer concentration (>5 %) [1, 2, 4]. As observed earlier, the convoluted FTIR spectra for F88 aqueous solution show a strong band in the range of 1,200–1,000 cm−1, separated in two peaks around 1,080 cm−1 and then other around 1,100 cm−1, which are assigned to C–O stretching vibration band of PEO block and PPO block, respectively [21, 22]. The cross-section of the two lines as shown in the spectra, determines the CMT.
The fluorescence profile featuring the I 1/I 3 ratio against temperature shows a CMT value in good agreement with the FTIR data. The CMT value obtained by both techniques is around 31.4 ± 0.5 °C. Figure 2b shows the differential thermal analysis (DSC) for 5 % aqueous solution of F88 in water and 2 M NaCl solutions. The presence of NaCl decreases the CMT value to 26.9 °C, as a consequence of the well-investigated salting-out effect of this electrolyte [12]. These results clearly indicate that temperature-induced micellization could be achieved, but much above the ambient temperature, which could be a drawback concerning a formulation treatment utilizing the formed nanostructures under ambient conditions. The observed results suggest molecularly dissolved F88 polymer chains which undergoes temperature induced aggregation. In order to investigate the possibility for realizing the nanostructures at ambient temperature, the interaction with alkanols was proposed, considering their varying solubility in the aqueous phase and the subsequent prospect for interaction.
Cloud Point (CP)
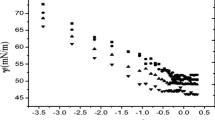
To analyze the effect of different alkanols on micellization, we carried out the cloud point (CP) measurements for 5 % F88 aqueous solutions in the presence of different alkanols (Fig. 3). CP measurement is an effective method to understand the phase separation and structural transition in block copolymer systems. The clouding phenomenon shows that the interactions between the PEO chains become increasingly attractive with increasing temperature in aqueous solution or in other words, water becomes a less good solvent. The clouding temperatures for 5 % F88 in water and 2 M NaCl as a function of alkanols with different chain lengths are shown in Fig. 3. The cloud-points of aqueous solutions of copolymers are related to the dehydration of PEO blocks with an increase in temperature. Ganguly et al. [23] have reported a temperature-induced sphere to rod structural transition for block copolymer micelles, which shifts to higher values in the presence of ethanol. The diffusion coefficient measurement of block copolymeric micelles in the presence of 1-butanol by Ding et al. [24] have shown an increase in micellar size with corresponding increase in alkanol concentration. Su et al. [25] observed the effect of 1-pentanol on micellar growth using fluorescence spectroscopy. Here, it can be clearly observed that the clouding temperature is significantly lowered on addition of alkanols with a longer carbon chain-length (>C4). It is expected that leaching out some water molecules from the PPO block and subsequent self-solubilization favors spherical aggregates, where alkanol will reside at the palisade layer between core and the coronal chains. The resultant introduction of hydrophobicity would increase the further solubilization and hence the cloud points are shifted to lower temperatures. The effect was significantly enhanced in the case of higher homologues of the carbon chain. The extent of influencing the clouding temperature was found to increase with a corresponding increase in the chain length of alkanol. The results were found in the order as 1-hexanol > 1-pentanol > 1-butanol. The CP for 5 % F88 is above 100 °C and hence not measurable. To our understanding, ethanol due to a higher water solubility would further hydrate the polymer blocks. As a result, the CP for aqueous F88 solutions in presence of ethanol were not measurable. To understand the role of ethanol, we tried to decrease the CP by addition of aqueous 2 M NaCl, a salt-out electrolyte. In agreement with our assumption, the aqueous solution of 5 % F88 in 2 M NaCl, showed a clear rise in the CP in the presence of ethanol due to increased solubility of the polymer blocks.
UV–Visible Spectroscopy
To investigate the effect of added alkanol on the micellization temperature of F88 aqueous solution, UV–visible measurements were carried out. Figure 4a shows the changes in CMTs for F88 in the presence of different alkanols. The results clearly correlate dependence of the carbon chain length on the CMTs of F88 solution. With increasing carbon chain length of the alkanol, the CMT value decreases almost proportionally. The observed trend is similar to the CP measurements, which showed that increased hydrophobicity of alkanol shifts the solubility of block copolymer molecules to lower values. Ethanol addition showed an increase in the CMT value, understood by the higher solubility of polymer blocks in aqueous phase. The measurements were extended to aqueous NaCl solutions to elucidate the effect of alkanol-induced micelle formation in the presence of structure making anion. The alkanol/PPO block interactions were enhanced as the polymer blocks were dehydrated by the presence of salting-out additive. Hence, further addition of alkanol would be more favorable due to facilitated hydrophobic interaction between the polymer blocks and alkanol chains. The trend for the lowering of the CMTs reveals the role of the hydrophobic chain of alkanol, which in turn promotes micellization. The addition of 0.1 % 1-octanol lowered the CMT value to 22.5 °C from initial value of 29.7 °C. Figure 4b shows the changes in absorbance as a function of temperature for different concentrations of added 1-butanol in 5 % F88 aqueous solutions. The intersection of the two lines in the plot gives the CMT value for the observed sample. It can be noted that with a gradual increase in the 1-butanol concentration, the observed CMTs are proportionally shifted to lower temperature values. The further addition of 1-octanol showed phase separation of the solution. We conclude that the PPO block of F88 and the alkanol interact with each other through hydrophobic interaction, which is vital to influence the micellization. We presume that micelles are composed of the PPO core and marginally extended PEO coronal chain, while the polar alkanols reside at the interface between the core and corona, the palisade layer. The obtained CMT values are shown in Table 2.
a CMTs of 5 % F88 aqueous solutions in different concentrations of alkanol. Open symbols (water), Closed symbols (2 M NaCl) squares ethanol, circles 1-butanol, triangles 1-pentanol, diamonds 1-hexanol, stars 1-octanol. b CMT plots for 5 % F88 aqueous solution in the presence of different concentrations of 1-butanol
Small Angle Neutron Scattering (SANS)
The accordingly formed micellar structure was investigated further using small angle neutron scattering. The formation of micelles and the aggregation number could be greatly affected by the presence of alkanol, both in water and aqueous salt solutions. In small angle neutron scattering experiments, one determines the differential scattering cross-section per unit volume as a function of the scattering vector Q, and for a monodisperse system of micelles it can be expressed as [20],
Here, ‘n’ denotes the number density of the micelles, P(Q) is the intraparticle structure factor and S(Q) is the interparticle structure factor. B is a constant term that represents the incoherent scattering background, which is mainly due to hydrogen in the sample. The block copolymer micelles can be considered as a spherical core–shell particle with different scattering length densities of the core and the shell. The structure of these micelles is described by following a model proposed by Pedersen and Grestenberg [26]. In this model, P(Q) contains four terms: the self-correlation of the core, the self-correlation of the chains, the cross term between core and chains, and the cross term between different chains. It is given by
where, N is the aggregation number of the micelle. b s and b c are excess scattering lengths of the core and chain, respectively. The values of b s and b c for core and chain of the block copolymer F88 have the values 400 × 10−12 and 227 × 10−12 cm, respectively. These values are obtained by considering the scattering lengths of EO, PO and D2O as 0.414 × 10−12, 0.331 × 10−12 and 1.914 × 10−12 cm, respectively. The volume of EO, PO and D2O was calculated as,
The calculated values were, 67.5, 96.3 and 30 Å3, respectively.
In order to describe the core radius (R c), we used a form factor of the monodisperse sphere as
For chains with Gaussian statistics in the shell of the micelles having a radius of gyration (R g) the form factor is defined as
where x = Q 2 R 2g . The core-chain interference term S sc is given by
The function ψ(Q, R g) is the form factor amplitude of the chain
Finally the chain–chain interference term S cc is given by
The structure factor S(Q) of the spherical micelles in Eq. (1) is calculated using the Percus–Yevick approximation for the case of hard sphere potential in the Ornstein–Zernike equation [27]. In the case of polydispersed micelles, Eq. (1) can be written as,
where f(R) is the size distribution and usually accounted by log normal distribution as given by
where R med and σ are the median value and standard deviation respectively. The mean and median values are related as R m = R med exp(σ2/2).
The mean core radius (R c), radius of gyration of the chain (R g), hard sphere radius (R hs), volume fraction of the micelles (ϕ) and polydispersity (σ) have been determined as the fitting parameters from the analysis. The aggregation number is calculated by the relation N = 4πR 3c /3v, where v is the volume of the surfactant monomer. Throughout the data analysis, corrections were made for instrumental smearing [28]. The parameters in the analysis were optimized by means of nonlinear least-square fitting program [29].
F88 being a highly hydrophilic polymer (containing 80 % of PEO), does not micellize in water, even up to a 5-wt% concentration of copolymer [30, 31]. Jain et al. [32] have reported the scattering function for 5 % F88 aqueous solutions at 30 °C which gives the radius of gyration, R g = 21 Å. A hydrodynamic radius of 29 Å has been reported for F88 unimers, determined using dynamic light scattering, by Brown et al. [30].
SANS profile for 5 % F88 aqueous solution in the presence of 1 % alkanols (C2, C4–C6) at 30 °C, is shown in Fig. 5a. The scattering profile for 5 % F88 in 1 % each of ethanol and 1-butanol, shows a very low intensity [32]. The tendency of ethanol to distribute more in the water phase will partly diminish the possibility of interaction with the hydrophobic PPO block [16]. The addition of 1-pentanol shows a considerable increase in the scattering profile indicating a higher degree of block/alkanol interactions responsible to influence the micellization. The appearance of a distinct correlation peak for addition of 1-hexanol depicts significant increase in the scattering intensity and improved micellization. 1-Butanol does improve micellization of a certain size, which is smaller than the ones for alkanols with a longer chain length. When this result is compared with the higher chain homologue, the role of the chain length could be understood, considering significant the micellization caused by 1-pentanol and 1-hexanol at much lower concentrations. Though, with increases in the chain length, a very marginal increase in the aggregation number could be noticed. We assume that gradual addition of a respective alcohol would increase the micellization, but will not interfere with the morphology to any extent, where it can cause shape transitions. Considering this idea, data were analyzed as a spherical shape model of the micellar structures. The results obtained clearly support the assumption made from the CMT data, which indicates the significant role of the carbon chain-length for a respective alkanol on the micellization temperature. The higher chain-length species is hence responsible for enhancing the hydrophobic effects, which replicates in increased aggregation number and scattering intensity, as observed for the SANS curves. The addition of a successive amount of alkanol further increases the aggregation number for 1-hexanol as shown in Fig. 5b. The gradual increase in 1-hexanol addition improves the aggregation number as obtained by the modeling of the respective SANS curve, a clear agreement to a successive decrease in CMT with a gradual increase in the amount of added alkanol as observed by the UV–Vis data. The increase in aggregation number was demonstrated, confirming the role of a higher chain length alkanol to trigger and then influence the micellization further. The respective solubility of alkanols in water is the defining parameter to influence the micellization of block copolymer solutions. Ethanol is completely soluble in water, while 1-butanol, 1-pentanol and 1-hexanol have values of 9, 2.7 and 0.6 wt%, respectively [33]. Due to preferential partitioning in the hydrophobic part, higher chain (C4–C6) alkanols will interact with the dehydrated PPO blocks and become solubilized to orientate at the palisade layer of the micelles [33]. In other words, an entropically opposed solubilization of alkanols in the aqueous phase is responsible for the micellization of the block copolymer [34]. The core radius and aggregation number were found to increase with a corresponding increase in the chain length of the alkanols with the effect being highest for 1-hexanol. The higher values of the volume fraction for these micelles is attributed to the higher degree of chain hydration [35, 36]. Considering the DSC results in Fig. 2b, the addition of the salt-out additive and alkanol could be an interesting combination for hydrated polymer blocks. The scattering profile for 5 % F88 in 2 M NaCl in presence of 1 % alkanol (C2, C4–C6) at 30 °C is shown in Fig. 6. As observed earlier, the aqueous solution of 5 % F88 in 2 M NaCl contains unimers [30]. Here, the addition of alkanols induced micelles with a higher aggregation number and therefore a higher forward scattering for the SANS curves, compared to salt free solutions. The micellar parameters obtained are reported in Tables 3 and 4. Ganguly et al. [23] reported sphere to rod transitions in P123 micelles in the presence of ethanol and NaCl. A poorly water-soluble C14 diol in the presence of NaCl was found to induce micellar transition in the micelles, which was at much lower temperature than in the absence of NaCl [37]. Our conclusions are in agreement with a recent study by Parekh et al. [38] describing the role of varying chain length of alcohols to influence the micellar characteristics of hydrophobic block copolymers. The short-chain alkanols showed demicellization, while improved micellar characteristics could be noticed for alcohols with longer chains. Finally we conclude that he effect of alkanol on micellization follows the sequence: ethanol < 1-butanol < 1-pentanol < 1-hexanol. The presences of alkanol induce micellization of 5 % F88 in water and the effect seems to be enhanced in the presence of NaCl, though it is not drastic enough to cause any shape transition. Table 5 shows the parameters used for the modeling of the SANS data.
a SANS profile for 5 % F88 in water in presence of 1 % alkanol at 30 °C. Open squares ethanol, open triangles 1-butanol, filled inverted triangles 1-pentanol, open circles 1-hexanol. b SANS profile for 5 % F88 aqueous solution in the presence of alkanols at 30 °C. Open triangles 4 % 1-butanol, open circles 2 % 1-hexanol, filled circles 4 % 1-hexanol
Conclusions
F88, a hydrophilic copolymer(EO103PO39EO103) containing 80 % PEO, remains as a unimer at ambient temperature. The micellization was induced in 5 % F88 solution in water and 2 M NaCl in the presence of alkanols (C2, C4–C6, C8). The results were investigated using spectral (FTIR, fluorescence), thermal (DSC), scattering (SANS) and cloud point methods. Alkanol decreases the CP and CMT to lower the temperatures by interacting with the PPO block of the copolymer. The effect was found to increase with increases in the chain length of alkanols. It can be concluded that alkanols behave in a similar manner to temperature by inducing the micellization. The presence of NaCl enhances the effect due to its salting out character. The aggregation parameters in the presence of alkanols were calculated using SANS.
The proposed interactions between the F88 block copolymer and different alkanols is illustrated in the Scheme 1.
The micellar parameters indicate an alkanol-assisted micellization and an increase in the aggregation number with increasing chain length. Though, alkanols influenced the micellization significantly, the effect was not strong enough to induce shape transition due to a higher PEO contribution in F88, which, in turn, is responsible for its hydrophilic nature. The results obtained will provide a better insight into solution behavior of F88 in the presence of an alkanols, which may be useful in tuning a certain formulation required for an industrial application point of view.
References
BASF Corp. (1989) Pluronic® and Tetronic® block copolymer surfactants. Technical Brochure
Nakashima K, Bahadur P (2006) Aggregation of water-soluble block copolymers in aqueous solutions: recent trends. Adv Colloid Int Sci 123:75–96
Tuzar Z, Kratochvil P (1993) Micelles of block and graft copolymers in solution. In: Matijevic E (ed) Surface and colloid science, chapter 1, vol 15. Plenum Press, New York, p 1
Chu B, Zhou Z (1996) Physical chemistry of polyoxyalkylene block copolymer surfactants. In: Mark Nace V (ed) Nonionic surfactants: polyoxyalkylene block copolymer studies, Chap. 3, vol 60. Marcel Dekker, USA, p 67
Chasin M, Langer R (1990) Pluronic®/PCL block copolymeric delivery system, drug and the pharmaceutical sciences, vol 3. Marcel Dekker, USA, p 71
Kabanov AV, Alakhov VY (2002) Pluronic® block copolymers in drug delivery: from micellar nano containers to biological response modifiers. Crit Rev Ther Drug Carrier Syst 19:1–75
Dwyer C, Viebke C, Meadows J (2005) Propofol induced micelle formation in aqueous block copolymer solutions. Colloid Surf A Physico Chem Eng Aspects 254:23–30
Nagarajan R (2001) Solubilization of “guest” molecules into polymeric aggregates. Polym Adv Technol 12:23–43
Hurter PN, Hatton TA (1992) Solubilization of polycyclic aromatic hydrocarbons by poly(ethylene oxide-propylene oxide) block copolymer micelles: effects of polymer structure. Langmuir 8:1291–1299
Collett JH, Tobin E (1979) Relationships between poloxamer structure and the solubilization of some para-substituted acetanilides. J Pharm Pharmacol 3:174–177
Causse J, Lagerge S, de Menorval L, Faure S, Fournel B (2005) Turbidity and 1H NMR analysis of the solubilization of tributylphosphate in aqueous solutions of an amphiphilic triblock copolymer. Colloid Surf A 252:51–59
Alexandridis P, Holwarth JF (1997) Differential scanning calorimetry investigation of the effect of salts on aqueous solution properties of an amphiphilic block copolymer (Poloxamer). Langmuir 13:6074–6082
Caragheorgheopol A, Caldararu H, Dragutan I, Joela H, Brown W (1997) Micellization and micellar structure of a poly(ethylene oxide)/poly(propylene oxide)/poly(ethylene oxide) triblock copolymer in water solution, as studied by the spin probe technique. Langmuir 13:6912–6921
Kipkemboi P, Fogden A, Alfredssen V, Flodstrom K (2001) Triblock copolymers as templates in mesoporous silica formation: structural dependence on polymer chain length and synthesis temperature. Langmuir 17:5398–5402
Feng P, Bu X, Pine DJ (2000) Control of pore sizes in mesoporous silica templated by liquid crystals in block copolymer-cosurfactant-water systems. Langmuir 16:5304–5310
Bharatiya B, Guo C, Ma JH, Hassan PA, Bahadur P (2007) Aggregation and clouding behavior of aqueous solution of EO–PO block copolymer in presence of n-alkanols. Eur Polym J 43:1883–1892
Gaisford S, Beezer AE, Mitchell JC (1997) Diode-array UV spectrometric evidence for cooperative interactions in binary mixtures of Pluronics F77, F87, and F127. Langmuir 13:2606–2607
Gaisford S, Beezer AE, Mitchell JC, Loh W, Finnie JK, Williams SJ (1995) Diode array UV spectrometric evidence for a concentration dependent phase transition in dilute aqueous solutions of Pluronic F87 (Poloxamer 237). J Chem Soc Chem Commun 18:1843–1844
Aswal VK, Goyal PS (2000) Small-angle neutron scattering diffractometer at Dhruva reactor. Curr Sci 79:947–953
Chen SH, Lin TL (1987) Methods of experimental physics, vol 23B. Academic Press, New York, p 489
Guo C, Liu HZ, Chen JY (1999) A Fourier transform infrared study of the phase transition in aqueous solutions of ethylene oxide-propylene oxide triblock copolymer. Colloid Polym Sci 277:376–381
Zana R (2000) Fluorescence studies of amphiphilic block copolymers in solution. In: Lindman B, Alexandridis P (eds) Amphiphilic block copolymers. Self-assembly and applications. Elsevier, Amsterdam, pp 221–234
Ganguly R, Aswal VK, Hassan PA, Gopalakrishnan IK, Yakhmi JV (2005) Sodium chloride and ethanol induced sphere to rod transition of triblock copolymer micelles. J Phys Chem B 109:5653–5658
Ding Y, Wang Y, Guo R (2003) Diffusion coefficients and structure properties in the Pluronic F127/n-C4H9OH/H2O System. J Disp Sci Technol 24:673–681
Su Y, Wei X, Liu H (2003) Influence of 1-pentanol on the micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymers in aqueous solutions. Langmuir 19:2995–3000
Pedersen JS, Grestenberg M (1996) Scattering form factor of block copolymer micelles. Macromolecules 29:1363–1365
Percus JK, Yevick GJ (1958) Analysis of classical statistical mechanics by means of collective coordinates. Phys Rev 110:1–13
Pedersen JS, Posselt D, Mortensen K (1990) Analytical treatment of the resolution function for small angle scattering. J Appl Cryst 23:321–333
Bevington PR (1969) Data reduction and error analysis for physical sciences. McGraw-Hill, New York
Brown W, Schillen K, Hvidt S (1992) Triblock copolymers in aqueous solution studied by static and dynamic light scattering and oscillatory shear measurements: influence of relative block sizes. J Phys Chem 96:6038–6044
Alexandridis P, Holtzwarth JF, Hatton TA (1994) Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: thermodynamics of copolymer association. Macromolecules 27:2414–2425
Jain NJ, Aswal VK, Goyal PS, Bahadur P (1998) Micellar structure of an ethylene oxide—propylene oxide block copolymer: a small-angle neutron scattering study. J Phys Chem B 102:8452–8458
Zana R (1995) Aqueous surfactant-alcohol systems: a review. Adv Colloid Inter Sci 57:1–64
Aswal VK, Kohlbrecher J (2006) Entropy-induced micellization of block copolymer in aqueous solution in presence of selective additives. Chem Phys Lett 425:118–122
Mortensen K (1996) Structural studies of aqueous solutions of PEO-PPO-PEO triblock copolymers, their micellar aggregates and mesophases; a small- angle neutron scattering study. J Phys Condens Matter 8:A103–A124
Mortensen K, Pedersen JS (1993) Structural study on the micelle formation of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymer in aqueous solution. Macromolecules 26:805–812
Bharatiya B, Aswal VK, Hassan PA, Bahadur P (2008) Influence of a hydrophobic diol on the micellar transitions of Pluronic P85 in aqueous solution. J Colloid Inter Sci 320:452–459
Parekh P, Singh K, Marangoni DG, Bahadur P (2012) Effect of alcohols on aqueous micellar solutions of PEO-PPO-PEO copolymers: a dynamic light scattering and 1H NMR study. J Mol Liq 165:49–54
Acknowledgments
PB thanks UGC, New Delhi for financial assistance [37-527/2009(SR)].
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Parmar, A., Bharatiya, B., Patel, K. et al. Alkanol-Induced Micelles of a Very Hydrophilic EO–PO–EO Block Copolymer: Characterization by Spectral and Scattering Methods. J Surfact Deterg 16, 105–114 (2013). https://doi.org/10.1007/s11743-012-1365-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-012-1365-9