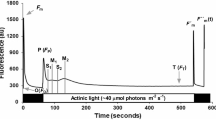
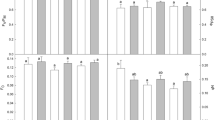
Abstract
The photosynthetic response was investigated on Chrysanthemum morifolium under dynamic light conditions in the 20–35 °C temperature range to evaluate the effect of climatic variables on photosynthetic induction. The plant material was grown under uniform, controlled conditions and its gas exchange was analyzed. The gas exchange measurements were used to investigate the rate of induction, momentary induction state, and the opening of stomata. At the varying temperature ranges and under dynamic light conditions, C. morifolium reached a quasi-steady-state induction equilibrium (ISeq(PAR,T)) within 14–45 min. For the same level of photosynthetically active radiation (PAR), the equilibrated level of steady-state induction increased as the temperature increased. It was highest approximately at 30 °C. The induction state was equilibrated at a lower level as the temperature increased to 35 °C. The interaction effect of PAR and temperature on induction state was not significant. The rate of photosynthetic induction and the time required at which the induction reached its 90 % value (t 90) was influenced by PAR significantly. The light history of a leaf had a significant effect on t 90, indicating that the time to reach a steady-state induction is different depending on the light environment and the period at which the leaf was exposed to light. The velocity of the photosynthetic induction was not affected by the temperature. It was associated with stomatal conductance of the leaf prior to the onset of light (g Sini).







Similar content being viewed by others
Abbreviations
- A low :
-
Photosynthetic rate obtained at PAR lower than saturating levels immediately after light fleck (μmol(CO2) m−2 s−1)
- A sat :
-
Light saturated photosynthetic rate (μmol(CO2) m−2 s−1)
- g Sini :
-
Initial stomatal conductance before the onset of light (mmol(CO2) m−2 s−1)
- IS(PAR,T) :
-
Light-, temperature-, and time-dependent induction (0 < IS(PAR,T) < 1)
- ISeq(PAR,T) :
-
Light- and temperature-dependent momentary induction equilibrium (0 < ISeq(PAR,T) < 1)
- IS60 :
-
Induction state 60 s after exposure to PAR (0 < IS60 < 1)
- PAR:
-
Photosynthetically active radiation (μmol m−2 s−1)
- T:
-
Temperature (°C)
- t 90 :
-
Time required to reach 90 % of ISeq(PAR,T) (min)
- τ:
-
Gradient constant that is dependent on time (s−1)
References
Bai KD, Liao D, Jiang D, Cao KF (2008) Photosynthetic induction in leaves of co-occurring Fagus lucida and Castanopsis lamontii saplings grown in contrasting light environments. Trees Struct Funct 22:449–462
Baker NR, Long SP (1986) Photosynthesis in contrasting environments. Elsevier Science Pub. Co., Amsterdam
Bretz F, Hothorn T, Westfall P (2010) Multiple comparisons using R. Chapman & Hall/CRC, Boca Raton
Bunce JA (2000) Acclimation of photosynthesis to temperature in eight cool and warm climate herbaceous C3 species: temperature dependence of parameters of a biochemical photosynthesis model. Photosynth Res 63:59–67
Bunce J (2008) Acclimation of photosynthesis to temperature in Arabidopsis thaliana and Brassica oleracea. Photosynthetica 46:517–524
Chazdon RL, Pearcy RW (1986) Photosynthetic responses to light variation in a rain-forest understory species. Am J Bot 73:726
Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y (2006) Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J Exp Bot 57:291–302
Jordan DB, Ogren WL (1984) The CO2/O2 specificity of Ribulose 1,5-bisphosphate carboxylase oxygenase-dependence on Ribulose bisphosphate concentration, pH and temperature. Planta 161:308–313
Kattge J, Knorr W (2007) Temperature acclimation in a biochemical model of photosynthesis: a reanalysis of data from 36 species. Plant Cell Environ 30:1176–1190
Kirschbaum MUF, Kuppers M, Schneider H, Giersch C, Noe S (1998) Modelling photosynthesis in fluctuating light with inclusion of stomatal conductance, biochemical activation and pools of key photosynthetic intermediates. Planta 204:16–26
Kirschbaum MUF, Oja V, Laisk A (2005) The quantum yield of CO2 fixation is reduced for several minutes after prior exposure to darkness. Exploration of the underlying causes. Plant Biol (Stuttgart, Germany) 7:58–66
Kuppers M, Schneider H (1993) Leaf gas exchange of beech (Fagus sylvatica L.) seedlings in lightflecks: effects of fleck length and leaf temperature in leaves grown in deep and partial shade. Trees (Berlin, West) 7:160–168
Leakey ADB, Scholes JD, Press MC (2005) Physiological and ecological significance of sunflecks for dipterocarp seedlings. J Exp Bot 56:469–482
Martin W, Scheibe R, Schnarrenberger C (2000) The Calvin cycle and its regulation. In: Leegood RC, Sharkey TD, von Caemmerer S (eds) Advances in Photosynthesis, Vol. 9. Kluwer Academic Publishers, pp 9–51
Medlyn BE, Loustau D, Delzon S (2002) Temperature response of parameters of a biochemically based model of photosynthesis. I. Seasonal changes in mature maritime pine (Pinus pinaster Ait). Plant Cell Environ 25:1155–1165
Naidu SL, DeLucia EH (1997) Acclimation of shade-developed leaves on saplings exposed to late-season canopy gaps. Tree Physiol 17:367–376
Naramoto M, Han Q, Kakubari Y (2001) The influence of previous irradiance on photosynthetic induction in three species grown in the gap and understory of a Fagus crenata forest. Photosynthetica 39:545–552
Naumburg E, Ellsworth DS (2000) Photosynthesis sunfleck utilization potential of understory saplings growing under elevated CO2 in FACE. Oecologia 122:163–174
Pearcy RW (1990) Sunflecks and photosynthesis in plant canopies. Annu Rev Plant Physiol Plant Mol Biol 41:421–453
Pearcy RW, Pfitsch WA (1994) Consequences of sunflecks for photosynthesis and growth of forest understory plants. Ecol Stud Anal Synth 100:343–359
Pinheiro J, Bates D (2009) Mixed-effects models in S and S-PLUS. Springer Science Business Media, Berlin
Pinto F, Berti M, Olivares D, Sierralta WD, Hinrichsen P, Pinto M (2011) Leaf development, temperature and light stress control of the expression of early light-inducible proteins (ELIPs) in Vitis vinifera L. Environ Exp Bot 72:278–283
Rabinowitch E (1957) Photosynthesis and energy transfer. J Phys Chem 61:870–878
Rijkers T, Vries P, Pons T, Bongers F (2000) Photosynthetic induction in saplings of three shade-tolerant tree species: comparing understorey and gap habitats in a French Guiana rain forest. Oecologia 125:331–340
Ritz C, Streibig JC (2008) Nonlinear regression with R. Springer Science & Business Media LLC, New York
Rosenqvist E (2001) Light acclimation maintains the redox state of the PSII electron acceptor Q(2) within a narrow range over a broad range of light intensities. Photosynth Res 70:299–310
Roux X, Grand S, Dreyer E, Daudet F (1999) Parameterization and testing of a biochemically based photosynthesis model for walnut (Juglans regia) trees and seedlings. Tree Physiol 19:481–492
Sage RF, Kubien DS (2007) The temperature response of C3 and C4 photosynthesis. Plant Cell Environ 30:1086–1106
Sassenrathcole GF, Pearcy RW (1992) The role of Ribulose-1,5-bisphosphate regeneration in the induction requirement of photosynthetic CO2 exchange under transient light conditions. Plant Physiol 99:227–234
Stegemann J, Timm HC, Kuppers M (1999) Simulation of photosynthetic plasticity in response to highly fluctuating light: an empirical model integrating dynamic photosynthetic induction and capacity. Trees Struct Funct 14:145–160
Tang YH, Liang NS (2000) Characterization of the photosynthetic induction response in a Populus species with stomata barely responding to light changes. Tree Physiol 20:969–976
Tang Y, Koizumi H, Satoh M, Washitani I (1994) Characteristics of transient photosynthesis in Quercus serrata seedlings grown under lightfleck and constant light regimes. Oecologia 100:463–469
Tausz M, Warren C, Adams M (2005) Dynamic light use and protection from excess light in upper canopy and coppice leaves of Nothofagus cunninghamii in an old growth, cool temperate rainforest in Victoria, Australia. New Phytol 165:143–156
Timm HC, Stegemann J, Kuppers M (2002) Photosynthetic induction strongly affects the light compensation point of net photosynthesis and coincidentally the apparent quantum yield. Trees Struct Funct 16:47–62
Urban O, Sprtova M, Kosvancova M, Tomaskova I, Lichtenthaler HK, Marek MV (2008) Comparison of photosynthetic induction and transient limitations during the induction phase in young and mature leaves from three poplar clones. Tree Physiol 28:1189–1197
Valladares F, Allen MT, Pearcy RW (1997) Photosynthetic responses to dynamic light under field conditions in six tropical rainforest shrubs occurring along a light gradient. Oecologia 111:505–514
Walcroft A, Whitehead D, Silvester W, Kelliher F (1997) The response of photosynthetic model parameters to temperature and nitrogen concentration in Pinus radiata D. Don. Plant Cell Environ 20:1338–1348
Walker DA (1973) Photosynthetic induction phenomena and the light activation of ribulose diphosphate carboxylase. New Phytol 72:209–235
Yamori W, Suzuki K, Noguchi K, Nakai M, Terashima I (2006) Effects of Rubisco kinetics and Rubisco activation state on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant Cell Environ 29:1659–1670
Young DR, Smith WK (1979) Influence of sunflecks on the temperature and water relations of two subalpine understory congeners. Oecologia 43:195–205
Zhang SB (2010) Temperature acclimation of photosynthesis in Meconopsis horridula var. racemosa Prain. Bot Stud 51:457–464
Zhang Q, Chen YJ, Song LY, Liu N, Sun LL (2012) Utilization of lightflecks by seedlings of five dominant tree species of different subtropical forest successional stages under low-light growth conditions. Tree Physiol 32:545–553
Zipperlen S, Press M (1997) Photosynthetic induction and stomatal oscillations in relation to the light environment of two dipterocarp rain forest tree species. J Ecol (Oxford) 85:491–503
Acknowledgments
This study was conducted under the “Intelligent Handling of Energy” project funded by The Region of Southern Denmark and EU (Reference: ERDFD-08-0021). We also wish to acknowledge financial support from Faculty of Agricultural Sciences, Aarhus University. In addition we wish to thank Can Hatipoglu, Detment of Mathematics, University of Porto for the suggestions on the mathematics.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Z. Gombos.
Rights and permissions
About this article
Cite this article
Ozturk, I., Ottosen, CO. & Ritz, C. The effect of temperature on photosynthetic induction under fluctuating light in Chrysanthemum morifolium . Acta Physiol Plant 35, 1179–1188 (2013). https://doi.org/10.1007/s11738-012-1157-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1157-x