Abstract
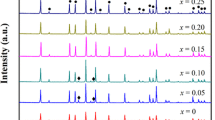
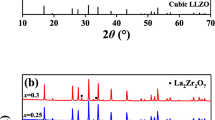
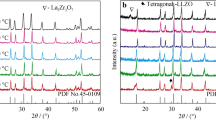
Garnet-like Li6.8La3Zr1.8Bi0.2O12 (LLZBO) + x mol.% Al2O3 (x = 0, 1.25, 2.50) lithium ionic electrolytes were prepared by conventional solid state reaction method under two different sintering temperatures of 1000°C and 1100°C. XPS, induced coupled plasma optical emission spectrometer (ICP-OES), XRD and AC impedance spectroscopy were applied to investigate the bismuth valance, lithium concentration, phase structure and lithium ionic conductivity, respectively. Electrical measurement demonstrated that ionic conductivity of Al-added LLZBO compounds could be obviously improved when the sample sintering temperature increased from 1000°C to 1100°C. The highest ionic conductivity 6.3×10-5 S/cm was obtained in the LLZBO-1.25%Al sample sintered at 1100°C, in consistent with the lowest activation energy 0.45 eV for the lithium ion migration. The mechanism related with good ionic conductivity in the Al-added LLZBO sample was attributed to the lattice distortion induced by the partial Al substitution at Zr sites, which is helpful to improve the migration ability of Li ions in lattice.
Similar content being viewed by others
References
Thangadurai V, Narayanan S, Pinzaru D. Garnet-type solid-state fast Li ion conductors for Li batteries: critical review. Chemical Society Reviews, 2014, 43(13): 4714–4727
Zhu J, Xu Z, Lu B G. Ultrafine nanoparticles decorated NiCo2O4 nanotubes as anode material for high-performance supercapacitor and lithium-ion battery applications. Nano Energy, 2014, 7: 114–123
Zhu J, Chen L B, Xu Z, et al. Electrospinning preparation of ultralong aligned nanofibers thin films for high performance fully flexible lithium-ion batteries. Nano Energy, 2015, 12: 339–346
Thangadurai V, Weppner W. Effect of sintering on the ionic conductivity of garnet-related structure Li5La3Nb2O12 and In-and K-doped Li5La3Nb2O12. Journal of Solid State Chemistry, 2006, 179(4): 974–984
Murugan R, Thangadurai V, Weppner W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angewandte Chemie International Edition, 2007, 46(41): 7778–7781
Thangadurai V, Kaack H, Weppner W. Novel fast lithium ion conduction in garnet-type Li5La3M2O12 (M = Nb, Ta). Journal of the American Ceramic Society, 2003, 86(3): 437–440
Awaka J, Kijima N, Hayakawa H, et al. Synthesis and structure analysis of tetragonal Li7La3Zr2O12 with the garnet-related type structure. Journal of Solid State Chemistry, 2009, 182(8): 2046–2052
Rangasamy E, Wolfenstine J, Sakamoto J. The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2O12. Solid State Ionics, 2012, 206: 28–32
Hubaud A A, Schroeder D J, Key B, et al. Low temperature stabilization of cubic (Li7–x Al x/3)La3Zr2O12: role of aluminum during formation. Journal of Materials Chemistry A: Materials for Energy and Sustainability, 2013, 1(31): 8813–8818
Wang X P, Xia Y, Hu J, et al. Phase transition and conductivity improvement of tetragonal fast lithium ionic electrolyte Li7La3Zr2O12. Solid State Ionics, 2013, 253: 137–142
Lee J M, Kim T, Baek S W, et al. High lithium ion conductivity of Li7La3Zr2O12 synthesized by solid state reaction. Solid State Ionics, 2014, 258: 13–17
Ahn J H, Park S Y, Lee J M, et al. Local impedance spectroscopic and microstructural analyses of Al-in-diffused Li7La3Zr2O12. Journal of Power Sources, 2014, 254: 287–292
Düvel A, Kuhn A, Robben L, et al. Mechanosynthesis of solid electrolytes: preparation, characterization, and Li ion transport properties of garnet-type Al-doped Li7La3Zr2O12 crystallizing with cubic symmetry. Journal of Physical Chemistry C, 2012, 116 (29): 15192–15202
Raskovalov A A, Il’ina E A, Antonov B D. Structure and transport properties of Li7La3Zr2–0.75x AlxO12 superionic solid electrolytes. Journal of Power Sources, 2013, 238: 48–52
He L X, Yoo H I. Effect of B-site ion (M) substitution on the ionic conductivity of (Li3x La2/3–x )1+y/2(MyTi1–y )O3 (M= Al, Cr). Electrochimica Acta, 2003, 48(10): 1357–1366
Kulkarni G U, Vijayakrishnan V, Rao G R, et al. State of bismuth in BaBiO3 and BaBi1–x PbxO3: Bi 4f photoemission and Bi L3 absorption spectroscopic studies. Applied Physics Letters, 1990, 57(17): 1823–1824
Xie H, Alonso J A, Li T, et al. Lithium distribution in aluminumfree cubic Li7La3Zr2O12. Chemistry of Materials, 2011, 23(16): 3587–3589
Boukamp B A. Equivalent Circuit, Users Manual. 2nd ed. The Netherlands: University of Twente, 1989, 1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, Y., Ma, L., Lu, H. et al. Preparation and enhancement of ionic conductivity in Al-added garnet-like Li6.8La3Zr1.8Bi0.2O12 lithium ionic electrolyte. Front. Mater. Sci. 9, 366–372 (2015). https://doi.org/10.1007/s11706-015-0308-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-015-0308-6