Abstract
The compounds CoNd2W2O10 and MnNd2W2O10 were prepared by solid state reaction between CoWO4, resp. MnWO4 and Nd2WO6. Intermediates were prepared in laboratory by a solid state reaction and by a co-precipitation reaction. The new single-phase compounds CoNd2W2O10 and MnNd2W2O10 were prepared only from intermediates prepared by solid state reaction, as in second case the morphology of particles is inappropriate and during the reaction an unknown phase (Nd–W–O) is formed. To describe the processes during thermal reactions, the DTA/TG were used. CoNd2W2O10 and MnNd2W2O10 belong to the orthorhombic crystallographic system, their diffraction pattern were indexed. The new compounds were tested as inorganic pigments. Application of the prepared compounds in ceramic glazes did not provide technological interesting results. The reason is probably the low thermal stability of the prepared compounds CoNd2W2O10 and MnNd2W2O10. In particle form CoNd2W2O10 has blue color and MnNd2W2O10 has brown color.
Similar content being viewed by others
Introduction
Rare earth metal tungstates and ditungstates, and cobalt and manganese tungstates have been widely studied for their catalytic, optical, magnetic and electrochemical properties (Barbosa et al. 2016; Sawicki et al. 2015; Tomaszewicz et al. 2013, 2016; Pan et al. 2013). Rare earth ions are characterized by incompletely filled 4f orbitals; they can absorb radiation in the visible region of the electromagnetic spectrum. Tungstate compounds can also be utilized as inorganic pigments due to their colorful or corrosion-inhibiting properties (Kalendova and Hajkova 2015; de Oliveira et al. 2008). Generally, MnWO4 and CoWO4 crystallize in the monoclinic system, as do most of the compounds RE2WO6 (RE = rare earth metal) (Chen and Weng 2016; Deng et al. 2012; Hu et al. 2010). A combination of MWO4 (M = Co, Mn, Cd, Cu) and RE2WO6 in a stoichiometric ratio very often leads to compounds of type MRE2W2O10, which crystallize in a monoclinic or orthorhombic system depending on the rare earth metal cation (Marrero-López et al. 2015; Tomaszewicz 2008 Tomaszewicz et al. 2009; Tomaszewicz and Kaczmarek 2010).
A solid state synthesis method is usually chosen for the preparation of MWO4 and RE2WO6, but there are other known methods, such as precipitation and hydrothermal methods resulting in nanoparticles (depending on synthesis conditions) or particles with other properties, such as high surface area or an amorphous structure (Barbosa et al. 2016; Blovská et al. 2013; Krustev and Ivanov 1992). The pigment with formula MnNd2W2O10 was prepared previously by solid state reaction in our laboratory. They were the synthesis condition determined and the color properties in particle form were tested (Blovská et al. 2013). This work is focused on a comparison of the effects of the synthesis methods of intermediates on the overall synthesis process and on the properties of the final products (pigments) CoNd2W2O10 and MnNd2W2O10, together with a study of the possibility of using the compounds mentioned as novel inorganic pigments for coloring glazes.
Experimental
Sample preparation
Pigments of the formula CoNd2W2O10 and MnNd2W2O10 were prepared by solid state reaction between intermediate compounds Nd2WO6 and MWO4 (M = Co, Mn).
Nd2WO6 was prepared by two methods: firstly by a solid state reaction technique at high temperature between Nd2O3 (99.9%, Sigma-Aldrich, CZ) and WO3 (99.9%, Osram, CZ); secondly by a co-precipitation reaction between Nd(NO3)3·6H2O (99.9%, Sigma-Aldrich, CZ) and NaWO4·2H2O (99.0%, Lach-Ner, CZ). In the case of the solid state reaction, the weighed amount of Nd2O3 was increased with regard to moisture and CO2 absorption by this oxide, resulting in partial formation of Nd2O2(CO3)2·2H2O during storage. The prepared mixture of Nd2O3 and WO3 was subjected to calcination in the following stages: 800 °C (6 h), 1000 °C (12 h) and 1050 °C (12 h).
Cobalt and manganese tungstates were prepared by calcination of the following mixtures: WO3 + CoSO4·7H2O (99.0%, Lach-Ner, CZ) and WO3 + MnSO4·H2O (99.0%, Lach-Ner, CZ), respectively. The starting materials were homogenized in a mortar mill (Fritsch), transferred to a corundum crucible and submitted to calcination in the following stages: 600 °C (6 h), 800 °C (12 h) and 1000 °C (12 h).
The mixtures of Nd2WO6 with MWO4 were prepared in the molar ratio 1:1. The Nd2WO6 + MWO4 (M = Co, Mn) mixtures in this molar ratio were homogenized, then calcined in the following stages: 900 °C (6 h), 1000 °C (6 h), 1050 °C (12 h) and 1100 °C (12 h) in an electric furnace, at a heating rate of 10 °C min−1. After each heating period the samples were gradually cooled to ambient temperature and manually ground (Blovská et al. 2013).
In the case of the precipitation method, 0.05 M solutions of individual compounds were prepared. The solution of neodymium, cobalt or manganese nitrate (Co(NO3)·6H2O, 99.1%, Lach-Ner, CZ; Mn(NO3)2·4H2O, 97.0%, Lach-Ner, CZ) was mixed with sodium tungstate solution with continuous magnetic stirring. The pH value of the solution was adjusted to 8.0 using 0.01 M NaOH (García-Pérez et al. 2012; Lei and Yan 2009). The precipitate was filtered, washed with distilled water and dried at 80 °C. The Nd2WO6 + MWO4·xH2O (M = Co, Mn) mixtures in proper molar ratio were homogenized in a mortar mill and calcined in the following stages: 900 °C (6 h), 1000 °C (6 h), 1050 °C (12 h) and 1050 °C (12 h) in an electric furnace at a heating rate of 10 °C min−1.
Materials characterization
The formation of the pigments with general formula MNd2W2O10 was followed by thermal analysis using an STA 449C Jupiter (NETZSCH) with simultaneous registration of TG and DTA curves in combination with phase analysis. The measurements were carried out in open ceramic crucibles, in air, between 30 and 1200 °C, the temperature increasing at 10 °C min−1; α-Al2O3 was used as a standard (Belina and Šulcová 2007). All heating processes of intermediate materials, with a special focus on MNd2W2O10 formation, are described in this work. The color of prepared final pigments was tested after application in ceramic glazes. Two types of ceramic glazes were used in this work. The first was a colorless lead ceramic glaze, 51% PbO (G028 91, Glazura, CZ) with thermal expansion coefficient α20–500 °C = 79.0.10−7 K−1, glazing temperature 880 °C. The second was an opaque white leadless zirconium glaze (Pw141 91, Glazura, CZ) with α20–500 °C = 59.5.10−7 K−1, glazing temperature 980 °C. An aqueous suspension containing 10 mass% of the pigment and 90 mass% of the frit was prepared for application to the ceramic body. The samples were measured in the visible region (400–700 nm) using a ColorQuest XE (HunterLab, USA). The measurement conditions were the following: illuminant D65, 10° complementary observed and measuring geometry d/8°. The color was described in terms of CIE L*a*b*. The values a* (the axis red–green) and b* (the axis yellow–blue) indicate the color hue. The value L* represents the lightness or darkness of the color in relation to the scale extending from white (L* = 100) to black (L* = 0). The value C (chroma) represents saturation of the color, calculated according to the formula: C = (a* 2 + b* 2)1/2. The color of pigment is also expressed by the hue angle H° defined by the angular position in the cylindrical color space (red H° = 0°–35°, orange H° = 35°–75°, yellow H° = 70°–105º, green H° = 105°–195°, blue H° = 195°–285° and violet H° = 285°–360°). The equation for calculation of the hue angle is H° = arc tg (b*/a*).
The new neodymium pigments were examined by powder X-ray diffraction (XRD). The X-ray diffraction patterns of the samples were obtained using a D8 Advance diffractometer (Bruker, GB) in the range 2θ of 10°–60° (step 0.02), CuKα radiation with scintillation detector. The wavelength of the radiation used was Kα1 = 0.15418 nm for angles 2θ < 35° and Kα2 = 0.15405 nm for angles 2θ > 35°.
Scanning electron microscopy (SEM) was used to study the morphology of intermediates of different origin (JEOL JSM-7500F, USA) in Gentle Beam Low mode.
The distribution of particle sizes of the calcined powders was obtained by laser scattering using a Mastersizes 2000 MU (Malvern Instruments, Ltd. GB). This is a highly integrated laser measuring system (He–Ne laser, λ = 633 nm) for analysis of particle size distribution. Fraunhofer approximation was used for evaluation of the particle size distribution (PSD). The PSD was measured using a flow system, with final pigments dispersed in water using ultrasound. Density determination were made by degassing the samples and hydrostatic weighing—pycnometric method, according to standard ČSN EN ISO 787-10.
Results and discussion
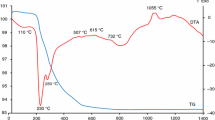
The simultaneous thermal analysis curves of CoWO4 and Nd2WO6 mixtures (Fig. 1) prepared by the solid state reaction method show only one slight exothermic effect, with a maximum at 1050 °C. Generally, this effect is connected with formation of ditungstate (Nd2W2O9) with the possibility of incorporation of MWO4 and new compound formation, in this case CoNd2W2O10. For the endothermic effect at 885 °C, connected with a mass loss of 0.11%, we have no reliable explanation. In the literature Tomaszewicz 2006 or Xiao et al. 2014 weight loss was associated to partial loss of oxygen by CoWO4 and the formation of CoWO4-x under inert atmosphere. However, for these arguments are not given the evidence, which is understandable, since weight loss is very small. In our case, the weight loss was observed although the measurement was performed under air atmosphere. An exothermic effect due to formation of ditungstate was observed in the DTA curve of the CoWO4 and Nd2WO6 mixture at 1042 °C (Krustev and Ivanov 1992; Pullar et al. 2007). The last significant endothermic peak (1182 °C) is connected with melting and decomposition of previously formed product.
DTA/TG curves obtained by measuring the thermal behavior of a CoWO4·xH2O and Nd2WO6 mixture, prepared as intermediates by the precipitation method, are recorded in Fig. 2. The first endothermic peak, with the minimum at 166 °C, relates to the slow loss of crystal water. From the value of weight loss 11.77% can be concluded that this is probably the loss of approximately two molecules of crystal water (CoWO4·2H2O) (Pullar et al. 2007). Nd2WO6 does not form hydrates, which was confirmed by DTA/TG measurement. The following exothermic peak, with a maximum at 409 °C, is connected to the crystallization of the amorphous form of CoWO4 obtained by the precipitation process. The amorphous form was confirmed by XRD analysis of dried intermediate product. The exothermic peak with a maximum at 582 °C is connected to the crystallization of compound probably containing Nd–W–O. A matching standard in the database PDF-4 (Powder Diffraction Files) was not found for this compound. The exothermic effect at 1039 °C represents formation of ditungstate Nd2W2O9. The endothermic peak at 1128 °C is associated with the melting of the mixture.
Figure 3 shows the thermal analysis (DTA/TG) record of the equimolar mixture of MnWO4·xH2O and Nd2WO6, prepared as intermediates by the precipitation method. Two endothermic peaks with minima at 168 and 244 °C are associated with the loss of one water molecule (mass loss 8.29%, MnWO4·H2O). The exothermic peak with a maximum at 582 °C is connected to the formation of an unknown phase containing Nd–W–O, as in the previous case. The exothermic effect with the maximum at 656 °C may be related to crystallization of the amorphous form of MnWO4. The exothermic peak with the maximum at 1028 °C is connected to the crystallization of Nd2W2O9. Melting and decomposition of the compounds present are represented by two endothermic peaks with minima at 1099 and 1121 °C.
Table 1 shows the results of XRD analysis for the samples obtained after the final heating period of mixtures CoWO4/Nd2WO6 and MnWO4/Nd2WO6, prepared by solid state reaction and precipitation. From the information in Table 1 it is evident that in the case of intermediate products prepared by a solid state reaction, the compounds were formed by mutual reaction (Fig. 4). The diffraction patterns of the new phases were subjected to an indexing procedure by FullProf_Suite Windows software. First successive diffraction lines recorded within 2θ of 10°–45° (CuKα) were selected for the indexing procedure. The results of indexing the diffraction patterns of CoNd2W2O10 and MnNd2W2O10 are presented in Table 3. Table 2 shows the values of parameters of the unit cells, as well as values of density of the new phases obtained experimentally and by calculation. (Pullar et al. 2007). The obtained phases are isostructural with MPr2W2O10 (M = Co, Mn) and crystallize in the orthorhombic system. Previous publications have reported that this type of compound may crystallize in the orthorhombic or monoclinic system depending on the rare earth element involved (Tomaszewicz 2008).
In Table 1 it was noted that when using precipitated intermediates, there was no formation of the expected ditungstate products. One of the explanations for this observation can be found in the morphology of the intermediates. Figure 5 shows the SEM images of CoWO4 prepared by solid state reaction (part A), CoWO4·xH2O prepared by precipitation (part B), Nd2WO6 prepared by solid state reaction (part C) and Nd2WO6 prepared by precipitation (part D). The intermediates prepared by precipitation exhibit crumb structure (part B and D in Fig. 5). Very small particles with a grain size of approximately 50–200 nm are very tightly coupled into larger units. The average particle sizes (d50) measured by laser diffraction were, for CoWO4, MnWO4 and Nd2WO6 prepared by solid state reaction, between 2.4 and 4.3 µm. The samples prepared by precipitation show a d50 value of between 28.6 and 32.6 µm. This is an indicator that very hard aggregates are produced by the precipitation method. The lower thermal stability of precipitated intermediates could be a second major reason why the new phase is not formed.
The color properties of samples prepared for evaluation of this type of compound as potential ceramic pigments are presented in Table 4. After the incorporation into colorless lead glaze G028 91, the compound CoNd2W2O10 has a dark blue hue, almost the same as in its powder form. Its coloring ability is not convenient, which shows in the second application, in opaque zirconium glaze Pw141 91. In the color space CIE L*a * b*, this sample is placed in the blue quadrant; the brightness value is inappropriately high, however. The same conclusion is also reached for the sample MnNd2W2O10, although in this case the color is brown. At this point it should be noted that due to the relatively low thermal stability of the final pigments, it is necessary to use a glaze with a low firing temperature.
Conclusions
Transition metal tungstates (CoWO4, MnWO4) and rare earth metal tungstates (Nd2WO6) may be prepared by a solid state reaction or a precipitation method. Hydrates with amorphous character were obtained using a precipitation method for sample preparation. The preparation of double tungstates with formulae CoNd2W2O10 and MnNd2W2O10 was carried out at temperatures of 1100 °C in the case of using intermediates prepared by reaction in the solid phase, and at 1050 °C in the case of using intermediates prepared by a precipitation method. Firing temperature values were determined based on data from thermal analysis (DTA/TG). Using this method all processes occurring during the firing of the reaction mixtures were identified. The new single-phase compounds CoNd2W2O10 and MnNd2W2O10 were prepared only from intermediates prepared by solid state reaction. The reason why there was no formation of the expected compound when using intermediates prepared by precipitation could be the unsuitable morphology of the particles. The results of thermal analysis and X-ray diffraction analysis also pointed to the formation of an unknown compound during the calcining of the mixture of precipitated intermediates, which prevented the formation of double tungstate. CoNd2W2O10 and MnNd2W2O10 belong to the orthorhombic crystallographic system. Application of the prepared compounds in glazes did not provide interesting results. The reason is probably the low thermal stability of the prepared compounds CoNd2W2O10 and MnNd2W2O10.
References
Barbosa HP, Kai J, Silva IGN, Rodrigues LCV, Felinto MCFC, Hölsä J, Malta OL, Brito HF (2016) Luminescence investigation of R3+—doped alkaline earth tungstates prepared by a soft chemistry method. J Lumin 170:736–742. doi:10.1016/j.jlumin.2015.07.014
Bělina P, Šulcová P (2007) Utilization of DTA for two step synthesis of Cu-Mn-Cr spinel. J Therm Anal Calorim 88:107–110. doi:10.1007/s10973-006-8100-6
Blovská V, Bělina P, Šulcová P (2013) Synthesis of tungstate pigments of the formula MNd2W2O10 (M = Ni, Zn, Mn). J Therm Anal Calorim 113:83–89. doi:10.1007/s10973-013-3129-9
Chen YC, Weng MZ (2016) Effect of sintering temperature and time on microwave dielectric properties of Nd2WO6 ceramics. J Ceram Soc Jpn 124:98–102. doi:10.2109/jcersj2.15155
de Oliveira ALM, Ferreira JM, Silva MRS, Braga GS, Soledale LEB, Aldeiza MAMM, Paskocimas CA, Lima SJG, Longo E, de Souza AG (2008) Yellow Zn x Ni1−x WO4 pigments obtained using a polymeric precursor method. Dyes Pigment 77:210–216. doi:10.1016/j.dyepig.2007.05.004
Deng JW, Chang L, Wang P, Zhang ED, Ma JM, Wang TH (2012) Preparation and magnetic properties of CoWO4 nanocrystals. Cryst Res Technol 9:1004–1007. doi:10.1002/crat.201200130
García-Pérez UM, Martínez-de la Cruz A, Peral J (2012) Transition metal tungstates synthesized by co-precipitation method: basic photocatalytic properties. Electrochim Acta 81:227–232. doi:10.1016/j.electacta.2012.07.045
Hu WB, Nie XL, Mi ZZh (2010) Controlled synthesis and structure characterization of nanostructured MnWO4. Mater Charact 61:85–89. doi:10.1016/j.matchar.2009.10.009
Kalendova A, Hajkova T (2015) Synthesis and investigation of the properties of tungstate-based anticorrosion pigments in coatings. Anticorros Methods Mater 62:307–321. doi:10.1108/ACMM-01-2014-1343
Krustev S, Ivanov K (1992) Preparation of manganous(II) tungstate by a precipitation method. J Alloy Compd 182:189–193. doi:10.1016/0925-8388(92)90587-Y
Lei F, Yan B (2009) Morphology-controlled synthesis, physical characterization and photoluminescence of novel self-assembled pomponlike white light Phosphor: Eu3−—doped sodium gadolinium tungstate. J Phys Chem C 113:1074–1082. doi:10.1021/jp8082634
Marrero-López D, Canales-Vásquez J, Ruiz-Morales JC, Núñez P (2015) High temperature properties of rare-earth tungstates RE2W2O9. J Alloy Compd 622:557–564. doi:10.1016/j.jallcom.2014.10.139
Pan L, Li L, Chen Y (2013) Synthesis and electrocatalytic properties of microsized AgWO4 and MWO4 (M = Co, Mn). J Sol Gel Sci Technol 66:330–336. doi:10.1007/s10971-013-3014-9
Pullar RC, Farrah S, Alford NMN (2007) MgWO4, ZnWO4, NiWO4 and CoWO4 microwave dielectric ceramics. J Eur Ceram Soc 27:1059–1063. doi:10.1016/j.jeurceramsoc.2006.05.085
Sawicki B, Grón T, Tomaszewicz E, Duda H, Górny K (2015) Some optical and transport properties of a new subclass of ceramic tungstates and molybdates. Ceram Int 41:13080–13089. doi:10.1016/j.ceramint.2015.07.003
Tomaszewicz E (2006) Reactivity in the solid state between CoWO4 and RE2WO6 where RE = Sm, Eu, Gd. Thermochim Acta 447:69–74. doi:10.1016/j.tca.2006.05.002
Tomaszewicz E (2008) New praseodymium(III) and d-electron metals tungstates of the formula MPr2W2O10 (M = Mn Co, Cd). J Therm Anal Calorim 93:711–715. doi:10.1007/s10973-008-9133-9
Tomaszewicz E, Kaczmarek SM (2010) Reactivity in solid state between CdWO4 and RE2WO6 (RE = Y, Nd, Sm, Eu, Gd, Dy, Ho, Re and Lu). Rev Adv Mater Sci 23:88–96
Tomaszewicz E, Typek J, Kaczmarek SM (2009) Synthesis, characterization and thermal behaviour of new copper and rare-earth metal tungstates. J Therm Anal Calorim 98:409–421. doi:10.1007/s10973-009-0295-x
Tomaszewicz E, Fuks H, Typek J (2013) Synthesis, thermal stability and magnetic properties of novel cadmium praseodymium tungstate Cd0.25Pr0.50Ca0.25WO4 and its solid solution. Thermochim Acta 568:95–103. doi:10.1016/j.tca.2013.06.029
Tomaszewicz E, Dabrowska G, Filipek E, Fuks H, Typek J (2016) New scheelite-type Cd1-3x □ x Gd2x (MoO4)1−3x (WO4)3x ceramics—their structure, thermal and magnetic properties. Ceram Int 42:6673–6681. doi:10.1016/j.ceramint.2016.01.024
Xiao TD, Tan X, Yi M, Peng S, Yang J, Dai Y (2014) Synthesi of commercial-scale tungsten carbide-cobalt (WC/Co) nanocomposite using aqueous solutions of tungsten (W), cobalt (Co) and carbon (C) precursors. J Mater Sci Chem Eng 2:1–15. doi:10.4236/msce.2014.27001
Acknowledgements
This work was supported by Czech Science Foundation (GAČR), Project No. 16-06697S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bělina, P., Machalíková, V., Gorodylova, N. et al. A comparative study of the influence of the method of synthesis of intermediate products in the preparation of CoNd2W2O10 and MnNd2W2O10 and their color properties in ceramic glazes. Chem. Pap. 71, 1597–1603 (2017). https://doi.org/10.1007/s11696-017-0150-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0150-7